Introduction
The maternal-fetal interface is an exquisite example of physiological symbiosis — a place where vascular flow, metabolic signaling, and hormonal regulation choreograph the success of pregnancy. Yet, in conditions of gestational insulin resistance and hyperandrogenism, this balance falters. The result is impaired placental function, restricted fetal growth, and elevated risk for long-term metabolic disease in offspring.
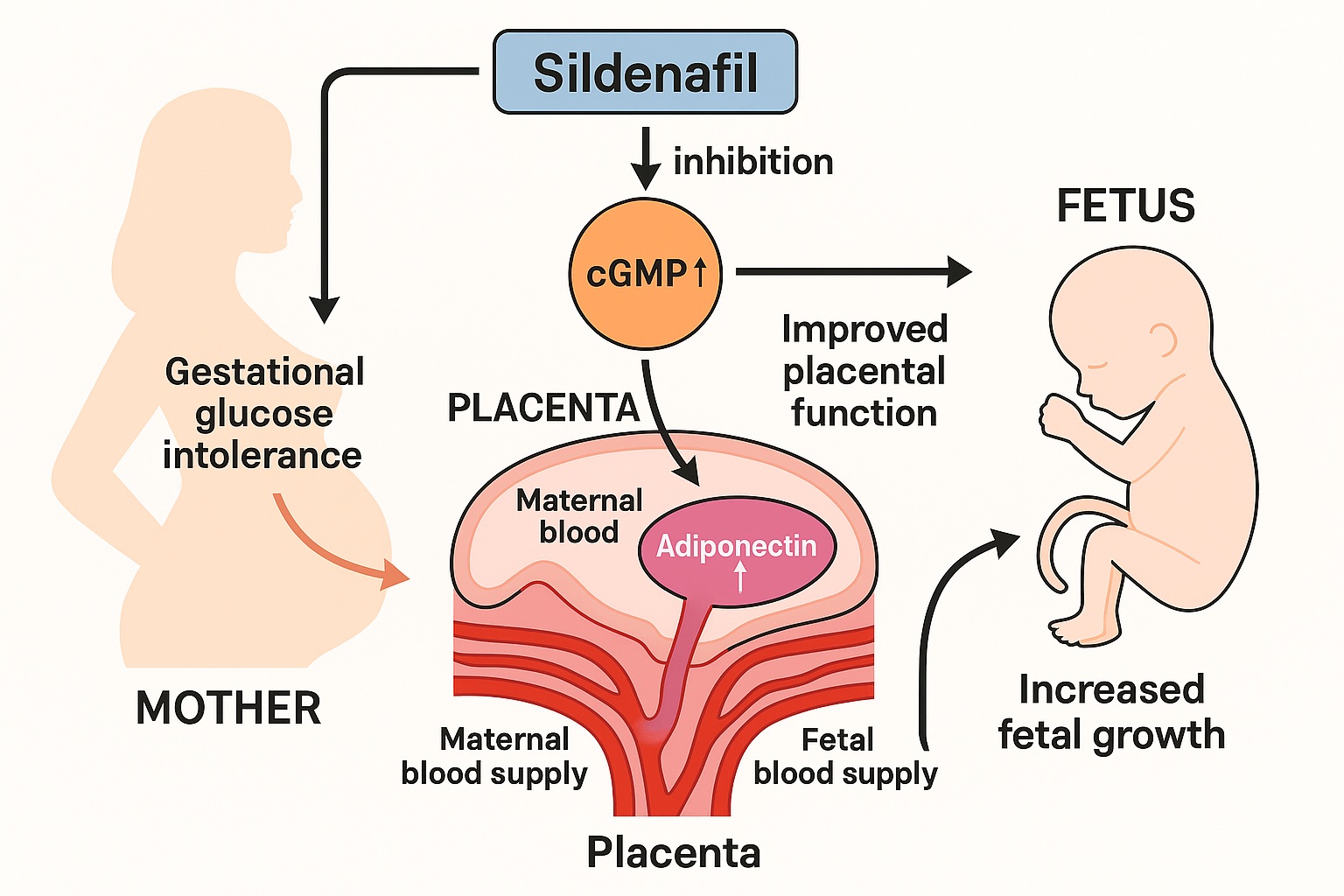
A recent experimental study investigating the effects of sildenafil citrate—better known as a phosphodiesterase type 5 (PDE5) inhibitor—has unveiled a fascinating metabolic dimension to this molecule. In gestational testosterone-induced glucose-intolerant rats, sildenafil not only improved placental vascular function and adipokine signaling but also enhanced fetal weight.
While sildenafil is traditionally associated with male erectile physiology, its pharmacological modulation of the NO–cGMP pathway offers broader systemic implications. This article explores the mechanistic and translational insights of these findings, highlighting how PDE5 inhibition could potentially recalibrate placental function under metabolic stress.
The Model: Testosterone, Gestation, and Metabolic Dysregulation
To simulate gestational glucose intolerance, researchers employed a testosterone-induced rat model, which mimics human gestational hyperandrogenism seen in disorders such as polycystic ovary syndrome (PCOS). Chronic exposure to elevated testosterone levels during pregnancy disrupts insulin signaling and vascular homeostasis, impairing nutrient transport across the placenta.
The resulting phenotype includes:
- Maternal glucose intolerance and insulin resistance
- Placental insufficiency, with reduced vascular perfusion
- Low fetal weight and delayed intrauterine growth
This experimental model therefore serves as a proxy for human conditions where metabolic and hormonal imbalances intersect with fetal growth restriction (FGR).
Within this context, sildenafil was evaluated not for its reproductive pharmacology, but for its endothelial and metabolic modulatory capacity.
Sildenafil and the NO–cGMP Pathway in Pregnancy
Sildenafil’s mechanism of action centers on phosphodiesterase type 5 (PDE5) inhibition, an enzyme responsible for degrading cyclic guanosine monophosphate (cGMP). Under physiological conditions, nitric oxide (NO) stimulates soluble guanylate cyclase (sGC) to convert GTP into cGMP. This second messenger triggers a cascade that relaxes vascular smooth muscle, improving blood flow and oxygen delivery.
In pregnancy, the placenta and uterine arteries rely heavily on this pathway. Impaired NO signaling—whether due to oxidative stress, hormonal imbalance, or endothelial dysfunction—leads to vasoconstriction and reduced perfusion, key drivers of fetal growth restriction.
By preventing cGMP degradation, sildenafil amplifies and prolongs NO-mediated signaling, restoring vascular tone and promoting enhanced placental perfusion.
This pharmacological effect lays the groundwork for the molecule’s secondary influence on metabolic signaling and fetal nutrient supply.
Placental Adiponectin: The Metabolic Mediator
Among the most intriguing findings of the study is sildenafil’s ability to upregulate placental adiponectin. Adiponectin, an adipokine primarily secreted by adipose tissue and, to a lesser extent, by the placenta, exerts profound metabolic and vascular effects.
In healthy pregnancies, adiponectin modulates insulin sensitivity, lipid metabolism, and angiogenesis within the placental microenvironment. However, in testosterone-induced glucose intolerance, placental adiponectin expression declines sharply, leading to endothelial dysfunction and suboptimal nutrient transfer.
Sildenafil administration reversed this trend, significantly increasing both placental adiponectin expression and fetal weight.
This observation suggests that the drug’s benefits extend beyond simple hemodynamic correction—it also rebalances endocrine signaling within the placenta.
Mechanistically, elevated cGMP levels may enhance AMP-activated protein kinase (AMPK) activity, a key metabolic regulator that promotes adiponectin synthesis while inhibiting oxidative stress. The result is a more metabolically resilient placenta, capable of supporting fetal growth despite maternal insulin resistance.
Oxidative Stress and Endothelial Dysfunction: The Hidden Culprits
Pregnancy represents a paradoxical oxidative state. While moderate oxidative stress is essential for placental angiogenesis and trophoblast differentiation, excessive free radical generation leads to endothelial damage and vascular rarefaction.
In testosterone-exposed rats, oxidative stress markers such as malondialdehyde (MDA) and nitrotyrosine were markedly elevated, correlating with reduced placental vascular density.
Sildenafil’s intervention produced a dual effect:
- It decreased oxidative stress by improving endothelial NO bioavailability.
- It upregulated antioxidant enzymes, including superoxide dismutase (SOD) and glutathione peroxidase (GPx).
These effects collectively restored the redox equilibrium, reducing peroxynitrite formation and improving microvascular flow.
In essence, sildenafil did not merely open vessels—it protected them from oxidative degradation, an essential component of sustained placental health.
Restoring Fetal Growth: The Downstream Effect
Perhaps the most clinically significant outcome of sildenafil administration was the augmentation of fetal weight.
Fetuses from testosterone-exposed mothers typically exhibit intrauterine growth restriction (IUGR) due to compromised placental perfusion and nutrient transport. Following sildenafil treatment, fetal weights approached near-normal levels, accompanied by increased placental efficiency.
Several interrelated mechanisms explain this improvement:
- Enhanced uteroplacental blood flow via smooth muscle relaxation in spiral arteries
- Improved nutrient transfer secondary to higher vascular surface area and capillary density
- Restored insulin sensitivity within the placenta and fetal tissues
- Adiponectin-mediated metabolic modulation, improving glucose uptake and lipid utilization
These effects reflect a systemic realignment of maternal–placental–fetal physiology, rather than a single-pathway correction.
The Vascular Architecture of a Revitalized Placenta
Histological analyses in the study revealed sildenafil’s structural impact on placental morphology. In testosterone-treated rats, the labyrinthine zone—responsible for maternal-fetal exchange—appeared disorganized, with diminished capillary formation.
Sildenafil administration led to:
- Increased capillary branching and vessel diameter
- Improved syncytiotrophoblast integrity
- Enhanced glycogen storage and reduced lipid accumulation
These changes imply that sildenafil promotes placental angiogenesis and metabolic remodeling, aligning structure with function.
Such morphological restoration mirrors sildenafil’s established benefits in pulmonary hypertension, where vascular remodeling improves tissue oxygenation and systemic outcomes.
Molecular Mechanisms: The Adiponectin–NO–AMPK Nexus
At the molecular level, the interplay between adiponectin, nitric oxide, and AMPK forms the crux of sildenafil’s efficacy in this model.
Adiponectin activates endothelial nitric oxide synthase (eNOS) via AMPK-dependent phosphorylation, enhancing NO production and vascular relaxation.
Conversely, NO itself can stimulate adiponectin expression, establishing a positive feedback loop that sustains vascular and metabolic harmony.
Sildenafil, by elevating intracellular cGMP, reinforces this cross-talk:
- PDE5 inhibition → cGMP accumulation
- cGMP activation of protein kinase G (PKG) → eNOS phosphorylation
- Increased NO bioavailability → improved vascular tone and mitochondrial function
- Secondary upregulation of adiponectin → restored insulin sensitivity and trophoblast health
This interconnected network exemplifies metabolic-vascular symbiosis, a concept that may hold translational potential for treating pregnancy complications characterized by dual metabolic and vascular pathology.
Translational Implications: From Bench to Bedside
While this study was conducted in an animal model, its implications extend into the human clinical domain. Conditions such as gestational diabetes mellitus (GDM) and preeclampsia share mechanistic hallmarks with testosterone-induced metabolic dysfunction—namely, endothelial injury, oxidative stress, and impaired NO signaling.
Sildenafil’s potential benefits in these settings include:
- Restoring uteroplacental perfusion in cases of fetal growth restriction
- Improving placental endocrine function, particularly adiponectin balance
- Reducing oxidative burden and enhancing mitochondrial efficiency
Indeed, sildenafil has already been evaluated in several human clinical trials for placental insufficiency and preeclampsia. Although the STRIDER trial in humans yielded mixed results—largely due to timing, dosing, and population differences—the mechanistic evidence remains compelling.
The present findings refine this picture by suggesting that metabolic correction may be as crucial as vascular dilation in achieving clinical efficacy.
Cautionary Perspectives and Ethical Dimensions
Despite its promise, sildenafil’s application in pregnancy demands prudence.
Animal models, while mechanistically informative, cannot fully capture the complexity of human placental biology. The human placenta exhibits species-specific transporter systems and endocrine patterns, making direct extrapolation uncertain.
Furthermore, concerns have been raised regarding fetal safety following maternal sildenafil exposure, with isolated reports suggesting potential adverse neonatal outcomes. However, such findings are often confounded by underlying disease severity rather than drug toxicity per se.
Future clinical translation must therefore emphasize:
- Precise dosing and timing of administration
- Rigorous monitoring of fetal cardiac and vascular responses
- Ethical transparency in maternal-fetal clinical research
Sildenafil’s journey from erectile dysfunction therapy to a potential placental protector is scientifically captivating but must proceed with measured optimism.
Integrating Pharmacology with Placental Biology
This study underscores an emerging paradigm: pharmacological interventions once confined to vascular or reproductive contexts may exert multisystemic metabolic effects.
The placenta, a unique endocrine organ, represents both a barrier and a bridge — and sildenafil’s dual impact on vascular tone and metabolic signaling demonstrates how targeted molecular therapy can influence this delicate equilibrium.
Moreover, the upregulation of adiponectin places sildenafil at the intersection of endocrinology and obstetrics. By improving insulin sensitivity and endothelial communication, it aligns with broader strategies aimed at reducing transgenerational metabolic risk.
This insight may also inform other therapeutic explorations, including the use of PDE5 inhibitors in metabolic syndrome, gestational diabetes, and polycystic ovary-related infertility.
As evidence grows, sildenafil could emerge as a prototype for vascular-metabolic therapeutics that restore cellular dialogue rather than merely treating symptoms.
Conclusion
The exploration of sildenafil’s effects in testosterone-induced glucose-intolerant pregnancies reveals a nuanced narrative of vascular restoration, endocrine recalibration, and fetal recovery.
By enhancing placental adiponectin expression, reducing oxidative stress, and improving fetal growth, sildenafil transcends its reputation as a vasodilator to become a potential metabolic modulator in the maternal-fetal unit.
While further clinical validation is required, this study reinforces a vital concept in perinatal medicine: targeting endothelial and metabolic dysfunction together may yield more robust outcomes than addressing either in isolation.
In this sense, sildenafil offers not just a pharmacological tool, but a conceptual bridge between vascular physiology and metabolic harmony — one that could redefine future therapies for high-risk pregnancies.
FAQ
1. How does sildenafil improve fetal growth in glucose-intolerant pregnancies?
Sildenafil enhances uteroplacental blood flow by inhibiting PDE5, which increases cGMP and nitric oxide–mediated vasodilation. This improved perfusion, combined with elevated placental adiponectin, restores nutrient transport and fetal growth.
2. What is the significance of adiponectin in this process?
Adiponectin regulates insulin sensitivity, lipid metabolism, and vascular health. By upregulating adiponectin, sildenafil improves placental metabolic efficiency and reduces oxidative stress, enabling better fetal development.
3. Could sildenafil be safely used in human pregnancies?
Preclinical data are promising, but human trials have yielded mixed results. Safety concerns and species differences necessitate cautious, well-designed clinical studies before routine obstetric use can be considered.