Introduction: The Fragile Alliance Between Heart and Tumor
Modern oncology has redefined survivorship, transforming once-lethal malignancies into chronic, manageable diseases. Yet this victory carries a paradox. As cancer survival rises, so does the long shadow of cardiotoxicity—the uninvited companion of many chemotherapeutic and targeted agents.
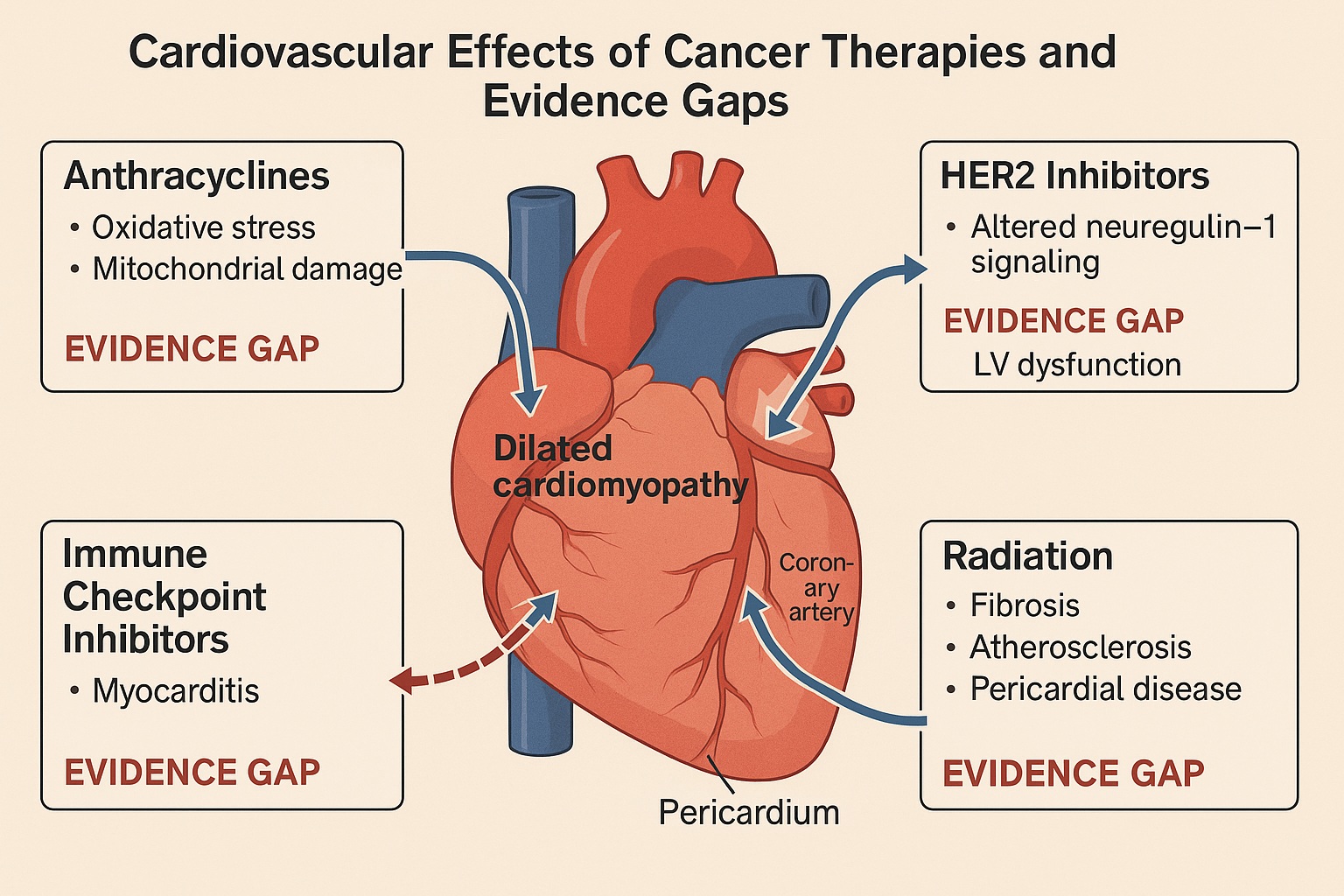
The heart, a tireless organ with a low tolerance for error, often pays the price of aggressive cancer therapy. Anthracyclines, HER2-targeted agents, immune checkpoint inhibitors, and even modern radiation techniques can compromise cardiac function. This complex overlap has given rise to a new discipline: cardio-oncology—a field tasked with protecting the cardiovascular system without compromising oncologic success.
The recent JACC: CardioOncology Expert Panel tackled a deceptively simple question: How do we balance cardiac safety and oncologic efficacy amid incomplete evidence? Their recommendations, drawn from interdisciplinary expertise, reveal a landscape rich in clinical promise but marred by striking knowledge gaps. This article distills and analyzes those insights, translating them into a coherent scientific narrative that bridges pharmacology, pathophysiology, and pragmatic clinical strategy.
The Expanding Burden of Cardiotoxicity in Cancer Care
The Price of Progress
The epidemiologic backdrop is sobering. Cardiovascular disease (CVD) and cancer now account for nearly 70% of global mortality. With the advent of precision oncology and immunotherapy, cancer survivors—once rare—now represent a rapidly growing population living long enough to develop treatment-related cardiovascular complications.
Anthracyclines remain archetypal offenders, inducing dose-dependent, irreversible myocardial damage through oxidative stress, mitochondrial dysfunction, and apoptosis. But the problem extends far beyond them. HER2-targeted agents like trastuzumab can cause reversible left ventricular (LV) dysfunction, while VEGF inhibitors, immune checkpoint inhibitors (ICIs), and proteasome inhibitors generate a spectrum of cardiotoxic syndromes—hypertension, myocarditis, arrhythmias, and heart failure.
The Diagnostic Dilemma
Despite our expanding pharmacologic arsenal, diagnostic definitions of cardiotoxicity remain inconsistent. Conventional parameters such as LV ejection fraction (LVEF) lack sensitivity for early myocardial injury. Global longitudinal strain (GLS) imaging, troponin assays, and natriuretic peptides offer incremental benefit but are unevenly implemented. The absence of standardized thresholds complicates both research interpretation and clinical decision-making.
The Underlying Paradox
Perhaps the greatest irony in cardio-oncology is that cardiotoxicity arises not from therapeutic failure but from therapeutic success. Agents potent enough to disrupt malignant signaling pathways often share molecular targets with cardiomyocytes and endothelial cells. The challenge, therefore, is not avoidance but precision mitigation—protecting cardiac integrity while preserving antitumor potency.
Pharmacologic Cardio-Protection: From Anthracyclines to HER2-Targeted Agents
The Anthracycline Legacy
Few drug classes have shaped the cardio-oncology landscape like the anthracyclines. Their efficacy in breast cancer, lymphoma, and sarcoma is undeniable, yet cumulative doses beyond 400–550 mg/m² of doxorubicin notoriously increase the risk of dilated cardiomyopathy.
Cardioprotection strategies have long centered on dexrazoxane, an iron-chelating agent that blunts free radical formation. The expert panel reaffirmed its benefit, particularly in high-dose or pediatric contexts. However, its use remains sporadic, hindered by lingering misconceptions about interference with chemotherapeutic efficacy—an assertion now largely disproven.
Other preventive approaches—liposomal anthracyclines and continuous infusion regimens—offer reduced myocardial exposure, yet remain underutilized due to cost and logistical barriers.
HER2-Targeted Therapies: Reversible but Relevant
HER2-directed agents such as trastuzumab revolutionized breast cancer therapy but introduced a new kind of cardiotoxicity—functional, non-structural, and potentially reversible. The pathophysiology involves disruption of neuregulin-1/ErbB2 signaling in cardiomyocytes, impairing cellular repair under stress.
The panel emphasized the importance of regular LV function monitoring every 3 months during therapy, noting that early discontinuation often allows recovery. However, evidence guiding the threshold for interruption and rechallenge remains weak. Emerging data suggest that “permissive cardiotoxicity”—temporarily tolerating mild LV decline under close monitoring—can be safe in selected patients, especially when oncologic urgency outweighs cardiac risk.
The New Agents: Mechanistic Mysteries
Targeted therapies and ICIs have introduced forms of cardiotoxicity not seen before. VEGF inhibitors provoke hypertension and microvascular dysfunction through endothelial nitric oxide suppression. Tyrosine kinase inhibitors (TKIs) like ponatinib and nilotinib can cause arterial occlusive events, while ICIs may trigger immune-mediated myocarditis, a rare but often fatal complication.
Given the heterogeneity of mechanisms, the panel advocated for mechanism-based risk stratification, emphasizing collaboration between oncologists, cardiologists, and pharmacologists. For instance, beta-blockers and ACE inhibitors show promise in mitigating subclinical LV strain reduction, but data remain fragmentary and require validation in prospective trials.
Surveillance Strategies: Balancing Science, Cost, and Clinical Sense
The Spectrum of Surveillance
Current practice oscillates between over-screening and neglect. The ideal surveillance protocol remains elusive because it must reconcile risk intensity, resource constraints, and patient burden.
The expert panel proposed a tiered approach:
- High-risk patients (e.g., anthracycline exposure >300 mg/m², prior radiation, pre-existing CVD) warrant echocardiography and biomarkers every 3 months.
- Intermediate-risk patients may undergo 6-month assessments.
- Low-risk groups might be monitored clinically, reserving imaging for symptom development.
Beyond LVEF: The Search for Sensitivity
The traditional reliance on LVEF is increasingly untenable. LVEF decline typically reflects late-stage injury; by the time it falls, myocyte loss is often irreversible. GLS imaging offers earlier detection, revealing subtle contractile impairment even with preserved ejection fraction.
Serum biomarkers such as high-sensitivity troponin and NT-proBNP provide complementary information on myocardial stress and injury. Integration of imaging and biomarkers into a multimodal algorithm may redefine how clinicians perceive “early warning signals” of cardiotoxicity.
Artificial Intelligence and Predictive Modeling
The future of cardio-oncologic surveillance lies in data integration. Machine learning models can synthesize longitudinal imaging, laboratory, and clinical data to predict cardiotoxicity risk before it manifests. Such predictive algorithms, however, require large, well-annotated datasets—something still lacking in the fragmented ecosystem of oncology research.
Permissive Cardiotoxicity: A Calculated Risk
The concept of permissive cardiotoxicity—continuing life-saving chemotherapy despite mild cardiac impairment—marks a paradigm shift. Historically, any reduction in LVEF mandated therapy discontinuation. However, the expert panel argues that in certain contexts, this rigidity may harm more than help.
In patients with aggressive malignancies and limited options, accepting manageable, reversible LV dysfunction can preserve oncologic efficacy without significantly compromising long-term outcomes. This approach demands meticulous follow-up, immediate initiation of cardioprotective medications, and readiness to withdraw therapy if function declines further.
Critics rightly caution that permissive cardiotoxicity risks normalizing injury, but in the absence of robust data, clinical judgment—anchored in multidisciplinary dialogue—remains the guiding compass.
Radiation and Novel Therapies: The New Frontier of Cardio-Oncology
Radiation-Induced Cardiotoxicity
Radiation, the oldest oncologic tool, continues to surprise us with its delayed cardiovascular consequences. Coronary artery disease, valvular dysfunction, pericarditis, and conduction abnormalities may emerge years after exposure, especially in breast and mediastinal cancers.
Modern techniques—intensity-modulated radiation therapy (IMRT) and proton beam therapy—reduce but do not eliminate risk. The panel recommends rigorous dosimetric planning, with mean heart doses kept below 5 Gy whenever feasible. Long-term surveillance, particularly for left-sided breast and Hodgkin lymphoma survivors, remains non-negotiable.
The Cardiotoxic Spectrum of Modern Oncology
Beyond radiation, the therapeutic horizon brims with immune and molecular agents whose cardiovascular footprints are only partially charted. Chimeric antigen receptor (CAR) T-cell therapy, for instance, can provoke cytokine release syndrome, causing myocardial stunning or arrhythmia. Similarly, BCR-ABL inhibitors, PARP inhibitors, and androgen receptor blockers each carry unique vascular or metabolic risks.
The expert panel calls for the creation of prospective pharmacovigilance registries, capturing real-world cardiovascular outcomes across diverse cancer populations. Without such infrastructure, the field risks remaining reactive rather than proactive.
Bridging Two Worlds: The Multidisciplinary Imperative
Collaboration as the Cornerstone
If cardio-oncology is to mature, it must dismantle silos. The expert panel’s most urgent recommendation is not technological but cultural: systemic integration of cardiologists and oncologists into a shared clinical ecosystem.
This collaboration extends beyond consultation. It involves co-designed care pathways, shared decision-making frameworks, and joint interpretation of diagnostic data. The concept of a “cardio-oncology tumor board,” where therapeutic decisions weigh both oncologic gain and cardiovascular risk, exemplifies this evolution.
Education and Infrastructure
The field’s rapid expansion has outpaced formal training. Few cardiology or oncology residencies include structured cardio-oncology curricula. The panel urges the establishment of dedicated fellowships and continuing medical education programs to cultivate the next generation of clinicians fluent in both molecular oncology and cardiovascular physiology.
From an infrastructural perspective, hospitals must invest in specialized cardio-oncology units equipped with advanced imaging, biomarker testing, and integrated electronic health records. Such platforms would allow real-time tracking of cardiac metrics throughout the cancer journey, facilitating early intervention.
The Research Deficit
Perhaps the most sobering revelation from the expert recommendations is the scarcity of high-quality randomized controlled trials (RCTs) addressing cardio-oncologic interventions. Most guidance currently rests on low- to moderate-certainty evidence, often extrapolated from small studies or surrogate endpoints.
Bridging this evidence gap demands coordinated funding initiatives and multicenter collaborations. Only through systematic data generation can cardio-oncology evolve from empiricism to evidence-based precision medicine.
From Evidence Gaps to Practical Wisdom
Redefining Success
The success of modern oncology should not be measured solely by tumor regression or survival curves. A life saved from cancer but compromised by heart failure or arrhythmia is an incomplete victory. Cardio-oncology’s mission is to transform that paradox into synergy—to enable patients not just to live longer, but to live well.
In practice, this requires a shift from episodic cardiology consultation to continuous cardiovascular stewardship throughout the cancer trajectory—from pre-therapy risk assessment to survivorship care.
The Ethical Equation
The absence of definitive data places clinicians in ethically ambiguous terrain. Should one prioritize oncologic control at the potential expense of cardiac health? Or safeguard the heart at the risk of undertreating cancer? The answer, the panel suggests, lies not in absolutes but in contextual proportionality—a nuanced balance of risks, values, and patient preferences.
The Future Landscape
Looking forward, cardio-oncology will likely expand its purview beyond prevention and surveillance into mechanistic intervention. Gene expression profiling, biomarker-guided drug dosing, and cardioprotective adjuncts tailored to molecular subtypes may soon render “cardiotoxicity” less of an inevitability and more of a controllable parameter. The intersection of omics technologies and precision pharmacology promises to make this vision tangible.
Conclusion: The Art of Dual Fidelity
The field of cardio-oncology stands on a threshold—no longer an afterthought, yet not fully a science of its own. The JACC Expert Panel reminds us that while our tools are imperfect, our responsibility is clear: to deliver therapies that preserve both life expectancy and life quality.
Filling the evidence gaps will require a collective commitment to multidisciplinary collaboration, standardized protocols, and prospective research. In the meantime, clinical wisdom—tempered by vigilance, compassion, and scientific humility—remains our most reliable instrument.
FAQ: Key Questions in Cardio-Oncology Practice
1. How can clinicians detect cardiotoxicity early during cancer therapy?
Early detection relies on integrating imaging (GLS, LVEF) and biomarkers (troponin, NT-proBNP) into structured surveillance protocols. High-risk patients should undergo assessments every 3 months, while lower-risk groups may be monitored semi-annually.
2. Is it ever acceptable to continue chemotherapy after mild cardiac dysfunction?
Yes, in selected cases under permissive cardiotoxicity principles. If LV impairment is mild and stable, continuing oncologic therapy with close cardiology supervision and cardioprotective medication can be justified, provided patient consent and careful monitoring.
3. What is the long-term goal of cardio-oncology research?
The ultimate goal is to develop predictive, personalized cardioprotection—using biomarkers, genomics, and imaging to anticipate toxicity and tailor interventions, ensuring every cancer survivor retains both cardiac and oncologic health.