Introduction
Premature ejaculation (PE) remains one of the most prevalent male sexual disorders, affecting up to 30% of sexually active men worldwide. Despite its frequency, it continues to challenge clinicians due to the interplay between psychological, neurobiological, and pharmacological determinants. The condition is characterized by ejaculation occurring sooner than desired, typically within one minute of vaginal penetration, accompanied by distress or interpersonal difficulty.
While selective serotonin reuptake inhibitors (SSRIs) such as paroxetine and sertraline have long been regarded as the cornerstone of pharmacologic therapy, the need for on-demand, well-tolerated, and rapidly acting options has led to the exploration of diverse pharmacologic agents. This includes tramadol, an atypical analgesic; sildenafil, a phosphodiesterase type 5 (PDE5) inhibitor; and topical anaesthetics, which act peripherally by modulating penile sensitivity.
The randomized, placebo-controlled clinical trial in focus investigated these agents’ on-demand efficacy and safety in men with lifelong premature ejaculation, offering valuable insights into the comparative mechanisms and clinical utility of four distinct pharmacologic pathways.
Study Overview and Clinical Framework
The trial enrolled adult men diagnosed with lifelong premature ejaculation, with an intravaginal ejaculation latency time (IELT) of less than one minute. Participants were randomly assigned to one of five groups:
- Group A: Tramadol 50 mg
- Group B: Sildenafil 50 mg
- Group C: Paroxetine 20 mg
- Group D: Topical lidocaine–prilocaine cream
- Group E: Placebo
Each medication was administered on-demand, one to two hours before intercourse, and patients maintained a structured sexual diary for IELT and subjective satisfaction assessment over several weeks. The double-blind, placebo-controlled design provided robust internal validity and enabled comparative pharmacodynamic evaluation.
The trial’s findings demonstrated superior efficacy of tramadol over other agents, with meaningful improvement in both IELT and sexual satisfaction, while maintaining tolerable adverse effects. The following sections analyze these results from a pharmacologic perspective.
Tramadol: Central Modulation of Ejaculatory Reflex Pathways
Mechanism of Action
Tramadol represents a centrally acting dual-mechanism analgesic, combining μ-opioid receptor agonism with serotonin and norepinephrine reuptake inhibition. This unique pharmacologic profile allows it to influence both spinal and supraspinal control of ejaculation.
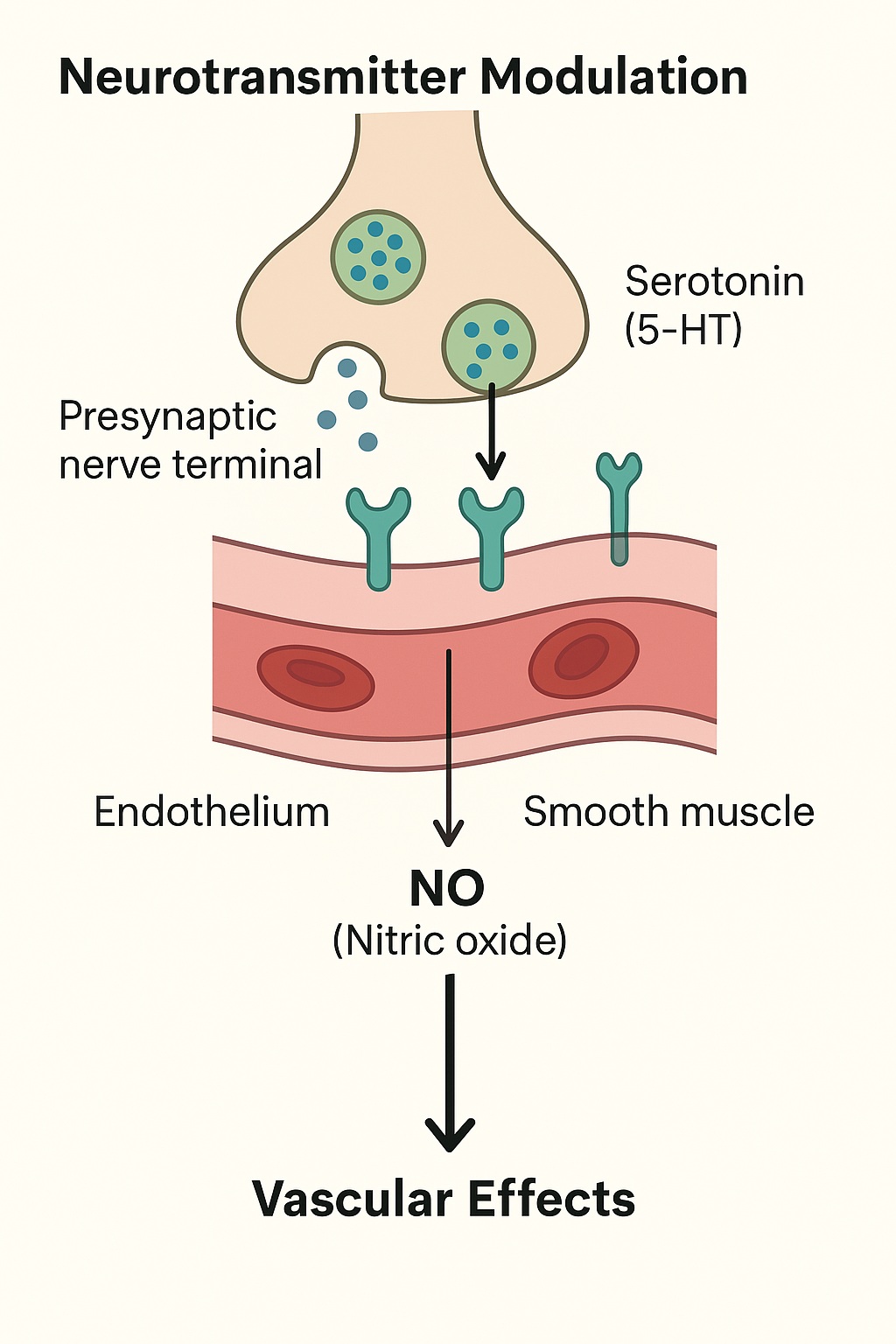
Ejaculation is orchestrated by the ejaculatory generator located within the lumbosacral spinal cord, integrating sensory inputs from the glans penis and higher cortical centers. Tramadol’s μ-opioid receptor activation dampens excitatory neurotransmission within this circuit, raising the threshold for ejaculatory reflex initiation. Simultaneously, serotonergic enhancement, particularly via 5-HT₂C and 5-HT₁A receptor modulation, delays emission by suppressing sympathetic outflow from the spinal cord.
Clinical Pharmacology and Kinetics
Tramadol exhibits rapid absorption, achieving peak plasma levels within two hours — aligning well with on-demand dosing. Its active metabolite, O-desmethyltramadol, is a potent μ-opioid agonist that contributes significantly to clinical effects.
By influencing both opioidergic and monoaminergic systems, tramadol bridges the pharmacologic domains of antidepressants and analgesics, producing an acute delay in ejaculation without the need for chronic administration — a distinct advantage over traditional SSRIs.
Clinical Efficacy
The study reported the greatest prolongation of IELT in the tramadol group, with mean increases four- to five-fold over baseline. Subjective measures of sexual satisfaction and control also improved significantly.
This efficacy likely reflects tramadol’s dual modulation of peripheral sensory transmission and central ejaculatory control, offering both physiological and psychological benefits.
Adverse Effects and Safety Considerations
Common side effects included nausea, dizziness, and mild sedation, consistent with opioid pharmacodynamics. However, no cases of dependency or withdrawal were reported, possibly due to low-dose, intermittent use. Clinicians should still exercise caution, particularly in patients with a history of substance misuse or concurrent serotonergic therapy (risk of serotonin syndrome).
Sildenafil: Vascular Enhancement and Secondary Ejaculatory Delay
Mechanistic Rationale
Sildenafil’s therapeutic relevance in PE stems from its action on PDE5 inhibition and consequent elevation of cyclic guanosine monophosphate (cGMP) levels in penile smooth muscle. Although originally developed for erectile dysfunction, sildenafil indirectly influences ejaculation by reducing performance anxiety, enhancing erection rigidity, and facilitating multiple coital attempts — all of which improve ejaculatory control.
Furthermore, sildenafil-induced vasodilation within pelvic and spinal regions may modulate sensory input, potentially increasing the ejaculatory threshold through central desensitization mechanisms involving the NO–cGMP–PKG signaling axis.
Clinical Pharmacology
After oral administration, sildenafil is rapidly absorbed with peak plasma concentration at about one hour, consistent with the on-demand design. Its half-life of approximately 4 hours provides a therapeutic window sufficient for sexual activity.
Unlike tramadol or paroxetine, sildenafil’s effect is peripheral and hemodynamic, not neurotransmitter-based. This makes it particularly useful in patients with coexisting erectile dysfunction, where it enhances performance without directly suppressing ejaculation reflexes.
Clinical Outcomes
In the trial, sildenafil improved IELT moderately (approximately two-fold over baseline) — less than tramadol but significantly greater than placebo. Participants also reported enhanced confidence and reduced anxiety-related dysfunction. These psychological benefits likely contribute to its observed efficacy.
However, sildenafil did not significantly increase ejaculatory latency in normoerectile men, confirming that its mechanism is primarily supportive rather than directly inhibitory of ejaculation.
Safety Profile
Adverse events were predictable and mild, including facial flushing, nasal congestion, and transient headache. No serious cardiovascular complications occurred. Its benign safety profile makes sildenafil an appealing adjunctive therapy, especially when combined with agents targeting central serotonergic or opioid pathways.
Paroxetine: The Classical Serotonergic Delay Mechanism
Pharmacologic Basis
Paroxetine, a potent selective serotonin reuptake inhibitor (SSRI), delays ejaculation by enhancing synaptic serotonin availability within both central and spinal control centers. Increased serotonin modulates 5-HT₂C and 5-HT₁B receptors, inhibiting ejaculatory motor output from the spinal generator.
However, this mechanism requires time-dependent synaptic adaptation — chronic receptor desensitization and downstream second-messenger recalibration — to fully manifest. As a result, acute or on-demand administration provides limited benefit, in contrast to daily dosing.
Pharmacokinetics and Dosing Implications
Paroxetine has a mean half-life of 21 hours, with steady-state plasma concentrations achieved after approximately 7–10 days of daily dosing. Its lipophilic nature ensures extensive CNS penetration, but these same properties reduce its practicality for on-demand regimens due to delayed onset and prolonged residual effects.
Thus, while paroxetine remains effective as a daily preventive agent for lifelong PE, its acute administration—as tested in this trial—fails to exploit its pharmacodynamic potential.
Clinical Results
The trial demonstrated modest improvement in IELT (approximately 1.5-fold increase), inferior to tramadol and sildenafil. This was consistent with the expectation that on-demand SSRIs lack sufficient pharmacokinetic exposure to elicit full serotonergic inhibition of ejaculation.
Adverse Effects
Typical SSRI-related adverse events — drowsiness, decreased libido, and mild gastrointestinal upset — were observed, though transient. Importantly, none of these effects were severe enough to discontinue treatment. However, the sexual side effects inherent to SSRIs—notably delayed orgasm and reduced libido—may limit their acceptability for on-demand use.
Topical Anaesthetics: Peripheral Desensitization of Penile Afferents
Mechanism of Action
Topical anaesthetics, such as lidocaine–prilocaine (EMLA) cream, reduce premature ejaculation by blocking sodium channels in penile sensory afferents, thereby increasing the latency of action potential generation. This blunts tactile stimulation of the glans and coronal ridge — the primary sensory triggers for ejaculation.
Unlike the centrally acting agents, these compounds exert their effects locally, preserving systemic hemodynamics and neurotransmitter balance. The onset of action is rapid (15–30 minutes), making them ideal for on-demand use.
Clinical Efficacy
The trial reported significant improvements in IELT — approximately two- to three-fold prolongation compared to baseline — comparable to sildenafil and slightly less than tramadol. Patients reported improved control, though some noted reduced sensory pleasure or partner numbness if adequate pre-coital washing was not performed.
Topical anaesthetics therefore offer a mechanically straightforward solution, but lack the neuropsychological reinforcement that centrally acting drugs provide. They are best suited for patients who prefer non-systemic therapy or those with contraindications to systemic medications.
Safety and Tolerability
Adverse events were minor and reversible — local burning, mild irritation, or transient numbness. Proper dosing and removal before intercourse minimize partner transfer and discomfort. Systemic absorption is negligible, eliminating risk of cardiovascular or central toxicity.
Comparative Analysis: Integrating Mechanism and Efficacy
Mechanistic Spectrum
The studied agents represent distinct mechanistic domains of ejaculatory control:
| Mechanistic Class | Representative Drug | Primary Target | Mode of Action |
|---|---|---|---|
| Opioidergic / Serotonergic Modulator | Tramadol | μ-opioid receptors, 5-HT reuptake | Central inhibition of ejaculatory reflex |
| PDE5 Inhibitor | Sildenafil | PDE5 enzyme (cGMP degradation) | Enhanced erectile function and indirect delay |
| SSRI | Paroxetine | Serotonin transporter (SERT) | Central inhibition via serotonergic tone |
| Local Anaesthetic | Lidocaine–prilocaine | Voltage-gated Na⁺ channels | Peripheral desensitization of penile afferents |
Tramadol’s dual central modulation yielded the highest clinical efficacy, outperforming purely serotonergic (paroxetine) or peripheral (anaesthetic) strategies. Sildenafil’s intermediate efficacy underscores its supportive role rather than direct inhibition of ejaculation.
Clinical Outcomes in Context
In the trial, mean IELT improvements followed a clear gradient:
- Tramadol – strongest prolongation (up to 4–5× baseline)
- Topical Anaesthetic – moderate (2–3× baseline)
- Sildenafil – mild to moderate (2× baseline)
- Paroxetine (on-demand) – least improvement (~1.5× baseline)
These data suggest that acute modulation of monoaminergic and opioid signaling is more effective than either chronic serotonergic potentiation or peripheral desensitization alone.
Practical Clinical Implications
- Tramadol is the most effective on-demand pharmacologic intervention for lifelong PE but should be reserved for patients without opioid contraindications.
- Sildenafil is suitable for men with coexisting erectile dysfunction, providing dual benefit.
- Paroxetine, while pharmacologically sound, is better reserved for daily use regimens rather than acute administration.
- Topical anaesthetics offer a safe, non-systemic alternative with rapid onset but reduced sensory feedback.
The selection among these depends on etiology, comorbidities, and patient preference, with tramadol representing a balanced central-peripheral hybrid mechanism.
Mechanistic Interactions and Synergistic Potential
Interestingly, the pharmacologic mechanisms of these agents suggest potential synergy when combined rationally. For instance:
- Tramadol + Sildenafil: Central inhibition (via opioid and serotonin reuptake blockade) complemented by peripheral enhancement of erection and confidence.
- Topical Anaesthetic + SSRI: Combination of local desensitization with central inhibition could further prolong IELT without increasing systemic risk.
However, such regimens must be clinically validated to avoid additive adverse effects, particularly regarding serotonin excess (with tramadol + SSRI) or systemic absorption of anaesthetics.
Safety and Tolerability: Balancing Efficacy and Risk
Across all groups, adverse effects were mild and transient. The most clinically relevant safety considerations include:
- Tramadol: Risk of dependence or serotonin syndrome with chronic or combined serotonergic use.
- Sildenafil: Contraindicated with nitrates; monitor for hypotension or visual changes.
- Paroxetine: Sexual dysfunction, somnolence, and withdrawal with abrupt discontinuation.
- Topical Anaesthetics: Local irritation, partner transfer, and transient numbness.
From a pharmacovigilance standpoint, on-demand dosing mitigates many chronic toxicity concerns, positioning these agents as viable options when appropriately selected.
Conclusion
The comparative clinical trial demonstrates that on-demand tramadol provides the most robust improvement in intravaginal ejaculation latency and patient satisfaction, supported by a dual pharmacologic mechanism involving opioid receptor activation and serotonergic modulation.
Sildenafil offers moderate benefit, primarily through psychogenic and vascular support, while paroxetine underperforms in on-demand settings due to pharmacokinetic limitations. Topical anaesthetics remain a practical, peripherally acting alternative with minimal systemic risk.
In essence, the pharmacologic landscape of premature ejaculation treatment reflects a neurochemical hierarchy — from serotonin and opioid signaling at the central level to sodium channel blockade at the periphery. Tramadol’s cross-domain activity places it at the intersection of these mechanisms, offering superior efficacy when administered judiciously.
FAQ: Key Clinical Questions
1. Why is tramadol more effective than paroxetine for on-demand use?
Because tramadol’s dual mechanism (opioidergic and serotonergic) acts acutely to inhibit spinal ejaculatory reflexes, whereas paroxetine requires chronic administration for full serotonergic modulation.
2. Can sildenafil be used as monotherapy for PE?
Sildenafil modestly prolongs ejaculation but is best used when premature ejaculation coexists with erectile dysfunction, due to its indirect central and psychological benefits.
3. Are topical anaesthetics safe for regular use?
Yes, when used correctly. They act locally without systemic absorption, but users must remove residual cream before intercourse to avoid partner numbness.