Introduction
The study of natural aphrodisiacs has advanced from folklore to pharmacology, guided increasingly by molecular validation. Among the promising botanical candidates is Raphia vinifera (Arecaceae), a palm species traditionally used in West Africa for the management of male sexual weakness, infertility, and reduced libido. The present experimental investigation employed aqueous and methanolic seed extracts of R. vinifera to evaluate their influence on sexual performance in sexually experienced male rats.
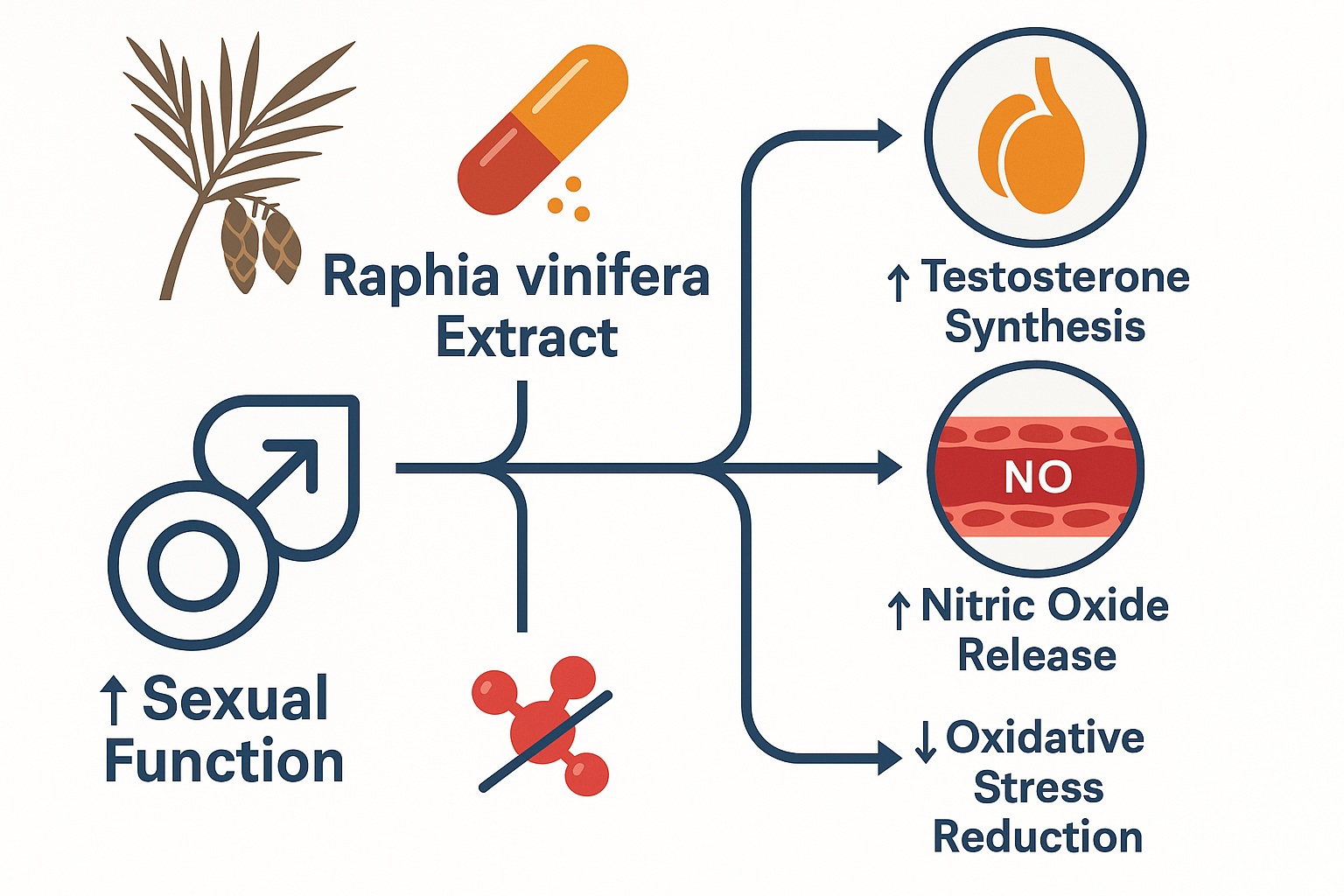
Rather than limiting itself to behavioral outcomes, the study explored hormonal and biochemical correlates, providing a foundation for mechanistic inference. The findings suggest that R. vinifera exerts multi-pathway aphrodisiac activity, potentially mediated through testosterone enhancement, nitric oxide (NO)-dependent vasodilation, and antioxidative protection of testicular tissue.
The following analysis dissects these mechanisms, integrating known molecular targets with observed pharmacodynamic outcomes.
Experimental Overview: Extract Preparation and Model Design
The experimental protocol utilized both aqueous and methanolic extracts of R. vinifera seeds, reflecting polarity-based extraction strategies aimed at maximizing phytochemical diversity. Methanol typically solubilizes polyphenols, flavonoids, and steroidal saponins — compounds associated with androgenic or vasodilatory activity. Conversely, the aqueous extract captures more hydrophilic agents such as carbohydrates and amino acid derivatives that may serve as metabolic co-factors in testosterone synthesis.
Adult sexually experienced male Wistar rats were divided into control and treatment groups, receiving graded doses of the extracts for several days. Behavioral parameters including mount latency (ML), intromission latency (IL), mount frequency (MF), intromission frequency (IF), and ejaculatory latency (EL) were recorded. Hormonal assays for serum testosterone, along with testicular biochemical analyses, supplemented these observations.
The study’s design, while straightforward, allowed differentiation between central neuroendocrine effects and peripheral testicular or endothelial mechanisms — a distinction critical for mechanistic understanding.
Phytochemical Profile: Clues to Mechanistic Potency
Preliminary phytochemical screening revealed the presence of flavonoids, saponins, alkaloids, tannins, steroids, and glycosides. Each of these compound classes contributes to sexual pharmacodynamics through distinct molecular actions.
- Flavonoids (e.g., quercetin-like compounds) act as potent antioxidants, scavenging reactive oxygen species (ROS) that impair Leydig cell steroidogenesis.
- Saponins, structurally similar to cholesterol, serve as precursors or modulators of steroid hormone biosynthesis, facilitating testosterone production.
- Alkaloids may influence dopaminergic or adrenergic neurotransmission, promoting central arousal and libido.
- Steroidal compounds can directly interact with androgen receptors or upregulate enzymes in the steroidogenic cascade.
Thus, the extract’s phytochemical composition provides a plausible foundation for the observed pharmacological profile: a multi-targeted enhancement of sexual motivation, erection, and performance.
Behavioral Pharmacology: Translating Sexual Reflexes into Neuroendocrine Signaling
Latency and Frequency Indices
Decreased mount and intromission latencies (ML and IL) signify enhanced sexual motivation and libido, likely mediated by dopaminergic activation within the medial preoptic area (MPOA) of the hypothalamus. This neuroanatomical center integrates sensory, hormonal, and psychological signals governing male sexual behavior.
Increased mount and intromission frequencies (MF and IF) suggest improved copulatory vigor, reflecting not merely libido but erectile maintenance and ejaculatory coordination — processes intricately linked to testosterone and endothelial nitric oxide synthase (eNOS) activity.
Prolongation of ejaculatory latency (EL) implies delayed climax, consistent with improved ejaculatory control or increased sexual endurance, a pattern frequently associated with elevated serum testosterone and improved cavernosal hemodynamics.
Central Neuroendocrine Activation
Behavioral enhancements following R. vinifera extract administration likely stem from activation of dopaminergic D2 receptors, which enhance luteinizing hormone (LH) release from the pituitary. LH stimulates Leydig cells in the testes to synthesize testosterone, thereby linking central neurotransmission with peripheral steroidogenesis.
This neuroendocrine integration supports the hypothesis that R. vinifera does not act solely at the periphery but also exerts central aphrodisiac effects, augmenting both sexual desire and physical performance.
Hormonal Modulation: Activation of the Hypothalamic–Pituitary–Gonadal (HPG) Axis
The study reported significant elevation in serum testosterone concentrations in extract-treated rats, particularly with the methanolic fraction. This observation underpins the primary endocrine mechanism of R. vinifera’s aphrodisiac effect.
Stimulation of Leydig Cells
Testosterone synthesis within Leydig cells is regulated by LH-mediated activation of adenylate cyclase, leading to cyclic adenosine monophosphate (cAMP) accumulation and subsequent activation of protein kinase A (PKA). PKA phosphorylates steroidogenic acute regulatory (StAR) protein, which facilitates cholesterol transport into mitochondria — the rate-limiting step of steroidogenesis.
It is plausible that bioactive saponins and flavonoids from R. vinifera enhance this pathway by either:
- Increasing LH receptor sensitivity,
- Providing cholesterol-like substrates for steroid biosynthesis, or
- Protecting Leydig cells from oxidative impairment, thus preserving StAR functionality.
Androgen-Dependent Behavioral Reinforcement
The testosterone surge induced by R. vinifera does not operate in isolation. Elevated androgen levels reinforce neural circuits governing sexual motivation, enhance nitric oxide synthase expression in penile tissue, and upregulate dopamine release within the hypothalamus — creating a positive feedback loop between endocrine and behavioral performance.
Nitric Oxide–Mediated Vasodilation: The Penile Endothelial Mechanism
One of the most critical physiological determinants of male sexual performance is the nitric oxide (NO)–cGMP pathway, which governs cavernosal smooth muscle relaxation and penile erection.
Mechanism of NO Generation
Sexual stimulation activates neuronal nitric oxide synthase (nNOS) in cavernous nerves and eNOS in endothelial cells, converting L-arginine into NO. The gas diffuses into adjacent smooth muscle cells, stimulating soluble guanylate cyclase (sGC) to produce cyclic guanosine monophosphate (cGMP). Elevated cGMP decreases intracellular calcium, leading to smooth muscle relaxation, arterial dilation, and increased penile blood inflow.
Modulation by Raphia vinifera Extract
Bioactive constituents of R. vinifera, particularly flavonoids, are known eNOS activators through the PI3K/Akt signaling pathway. This enhances endothelial NO production, mimicking or amplifying the physiological effects of phosphodiesterase type-5 inhibitors such as sildenafil.
Furthermore, the antioxidant capacity of the extract prevents NO degradation by reactive oxygen species, ensuring sustained bioavailability. Thus, R. vinifera acts both as an NO stimulator and stabilizer, improving penile vascular tone and erectile quality.
Comparison to Pharmacologic Agents
Unlike sildenafil, which prevents cGMP breakdown, R. vinifera appears to enhance upstream NO synthesis and protect against oxidative NO loss. This dual modulation distinguishes its pharmacodynamic profile and offers a potentially safer, more physiological mode of erection enhancement.
Oxidative Stress Mitigation: Protecting Testicular Function
Testicular function is exquisitely sensitive to oxidative stress, which damages spermatozoa, disrupts steroidogenesis, and impairs mitochondrial ATP generation. Chemically, the methanolic extract of R. vinifera exhibited robust antioxidant capacity, likely attributed to polyphenolic constituents.
Mechanistic Basis of Antioxidant Protection
Reactive oxygen species (superoxide anion, hydroxyl radical, hydrogen peroxide) can oxidize lipids in testicular membranes, leading to malondialdehyde (MDA) accumulation — a biomarker of lipid peroxidation. R. vinifera extract was shown to reduce MDA levels, signifying decreased peroxidative injury.
Concurrently, the extract enhanced superoxide dismutase (SOD) and catalase (CAT) activity, key components of the enzymatic antioxidant defense. This biochemical rebalancing maintains mitochondrial integrity in Leydig cells, supporting sustained testosterone synthesis.
Integration with Sexual Function
The oxidative protection of testicular tissue ensures functional preservation of spermatogenesis, androgen production, and sperm motility, aligning with improved sexual performance metrics. In essence, the antioxidant mechanism complements hormonal and vascular pathways to sustain male reproductive physiology.
Comparative Efficacy of Aqueous vs. Methanolic Extracts
While both extracts demonstrated aphrodisiac properties, the methanolic fraction consistently produced stronger effects on behavioral and biochemical endpoints. This aligns with the principle that methanol extracts lipophilic compounds — particularly steroidal saponins and flavonoids — which exert more potent endocrine and endothelial actions.
The aqueous extract, though milder, may contain synergistic hydrophilic antioxidants and amino acids that support overall metabolic resilience. Thus, the combined data suggest that R. vinifera’s efficacy derives from composite phytochemical synergy rather than a single active constituent.
Possible Molecular Pathways: Integrative Mechanistic Model
The pharmacodynamic convergence of hormonal, endothelial, and antioxidant pathways explains the multi-layered aphrodisiac effect of Raphia vinifera. A mechanistic model can be summarized as follows:
- Neuroendocrine Activation – Stimulation of hypothalamic dopaminergic tone increases LH secretion, initiating testicular steroidogenesis.
- Testosterone Enhancement – Saponins and flavonoids upregulate StAR and P450scc enzymes, elevating serum testosterone.
- Endothelial NO Upregulation – PI3K/Akt-mediated activation of eNOS increases NO release, facilitating penile vasodilation and erection.
- Antioxidant Protection – Flavonoids mitigate ROS, preserving NO stability and Leydig cell function.
- Feedback Reinforcement – Elevated testosterone and improved hemodynamics enhance libido and copulatory performance.
This integrated framework underscores R. vinifera as a multifactorial modulator of male sexual physiology, targeting the neuroendocrine, vascular, and oxidative dimensions of sexual function.
Translational Implications: From Animal Models to Human Pharmacology
While rodent models remain valuable for preclinical validation, translation to human physiology requires caution. Nonetheless, the mechanisms identified — particularly testosterone elevation and NO enhancement — correspond to well-characterized therapeutic targets in human sexual dysfunction.
Potential Clinical Application
In principle, R. vinifera–derived formulations could complement or serve as alternatives to conventional phosphodiesterase-5 inhibitors in cases of mild to moderate erectile dysfunction, particularly when associated with oxidative or hormonal imbalance. Its antioxidant properties may further protect against age-related testicular decline.
Safety and Toxicological Considerations
The study reported no overt toxicity or behavioral distress at therapeutic doses. However, chronic toxicity studies, genotoxicity assays, and endocrine profiling are necessary before human application. Herbal-derived androgenic stimulation can theoretically disrupt the HPG axis if used excessively, warranting controlled dosing regimens.
Limitations and Future Research Directions
The study’s findings, while compelling, invite further molecular and clinical exploration:
- Molecular Characterization: Identification of active compounds through LC–MS/MS and NMR spectroscopy.
- Target Validation: In vitro assays on Leydig and endothelial cells to confirm specific enzyme modulation (StAR, eNOS).
- Gene Expression Studies: Evaluation of mRNA expression for steroidogenic and antioxidant enzymes.
- Comparative Pharmacokinetics: Bioavailability assessment of aqueous vs. methanolic constituents.
- Clinical Translation: Controlled human trials assessing hormonal, vascular, and psychological outcomes.
Future work integrating omics-based approaches (metabolomics, transcriptomics) could unravel additional targets and support rational phytopharmaceutical development.
Conclusion
The aphrodisiac activity of Raphia vinifera seed extracts is not a matter of anecdote but of demonstrable biochemical synergy. The experimental evidence supports a tripartite mechanism encompassing:
- Endocrine stimulation — increased testosterone synthesis via activation of the HPG axis.
- Endothelial facilitation — enhanced nitric oxide release and improved penile hemodynamics.
- Antioxidant defense — reduction of oxidative stress, preserving testicular and vascular function.
By engaging these convergent molecular pathways, R. vinifera exerts a coordinated enhancement of libido, erection, and ejaculatory performance. Its pharmacologic profile suggests a natural prototype for integrative treatment of male sexual dysfunction, bridging traditional ethnomedicine with modern biochemical understanding.
FAQ: Scientific Clarifications
1. How does Raphia vinifera differ mechanistically from synthetic drugs like sildenafil?
While sildenafil inhibits PDE-5 to prevent cGMP breakdown, Raphia vinifera appears to act upstream — increasing NO synthesis and protecting its bioavailability, while simultaneously stimulating testosterone and antioxidant defenses.
2. Which phytochemicals are most responsible for the aphrodisiac effect?
The evidence points primarily to steroidal saponins (enhancing steroidogenesis) and flavonoids (boosting NO release and antioxidation), supported by minor contributions from alkaloids that influence central dopaminergic tone.
3. Can the observed effects be extrapolated to human males?
The underlying molecular pathways — testosterone synthesis, NO-mediated vasodilation, and oxidative stress reduction — are conserved across mammals. However, human pharmacokinetics and dosing require rigorous clinical validation before therapeutic use.