Introduction
Few obstetric complications have both fascinated and frustrated clinicians as much as preeclampsia — a hypertensive disorder of pregnancy characterized by systemic endothelial dysfunction, placental ischemia, and multiorgan involvement. Despite centuries of study, it remains one of the leading causes of maternal and perinatal morbidity and mortality, accounting for approximately 70,000 maternal and 500,000 neonatal deaths annually worldwide. Current therapy remains disappointingly primitive: control the blood pressure, prevent seizures, and deliver the fetus — often prematurely.
Against this grim background, sildenafil citrate, a selective phosphodiesterase type 5 (PDE5) inhibitor better known for its role in treating erectile dysfunction and pulmonary hypertension, emerged as an unexpected contender. The idea seemed elegant: improve uteroplacental blood flow by enhancing nitric oxide–mediated vasodilation, thereby alleviating fetal hypoxia and maternal hypertension. Early preclinical data were dazzling. Animal models showed improved placental perfusion, better fetal growth, and reduced oxidative stress. It appeared that sildenafil might finally offer a pharmacologic bridge to term in preeclamptic pregnancies.
Yet enthusiasm has since been tempered by reality. The translation from promising bench data to clinical safety has not been straightforward. The drug that symbolized vascular restoration in one setting became a symbol of scientific caution in another. The question is no longer “Can sildenafil help?” but rather “Should we still be enthusiastic?”
This article explores that question through a rigorous examination of the biological rationale, mechanistic insights, clinical evidence, and ethical debates surrounding sildenafil’s role in preeclampsia.
Preeclampsia: A Multifactorial Vascular Crisis
Preeclampsia is not a single disease but a syndrome arising from abnormal placentation and maladaptive maternal vascular response. The hallmark is inadequate trophoblastic invasion of spiral arteries during early gestation, leading to placental hypoperfusion and intermittent ischemia-reperfusion injury. This sets off a cascade of oxidative stress, inflammatory cytokine release, and antiangiogenic factor secretion, most notably soluble fms-like tyrosine kinase-1 (sFlt-1).
sFlt-1 acts as a decoy receptor for vascular endothelial growth factor (VEGF) and placental growth factor (PlGF), neutralizing their proangiogenic effects and crippling endothelial health. The result is systemic vasoconstriction, capillary leak, and microvascular dysfunction affecting the kidneys, liver, and brain — the defining pathophysiological triad of hypertension, proteinuria, and organ injury.
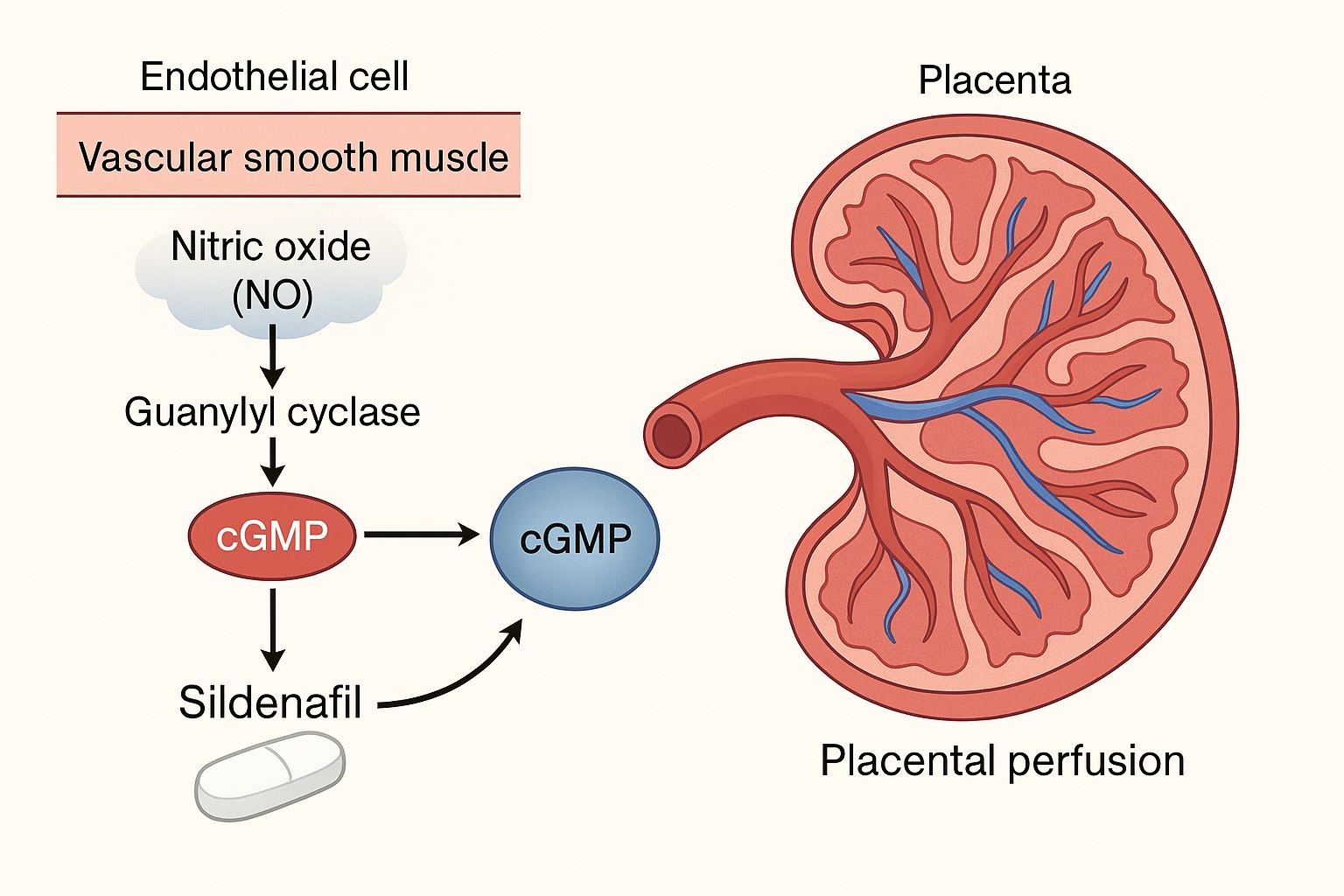
Central to this process is endothelial nitric oxide (NO) depletion. Under physiological conditions, NO produced by endothelial nitric oxide synthase (eNOS) diffuses into vascular smooth muscle cells, activating guanylyl cyclase and elevating cyclic guanosine monophosphate (cGMP) levels. cGMP, in turn, promotes smooth muscle relaxation and vasodilation. In preeclampsia, oxidative stress degrades NO and reduces cGMP availability, shifting the vascular tone toward constriction.
Thus, preeclampsia represents a failure of NO–cGMP signaling within a hostile inflammatory and antiangiogenic environment. Theoretically, restoring that pathway might restore balance. This is where sildenafil entered the stage.
Mechanism of Sildenafil: Revisiting the NO–cGMP Pathway
Sildenafil selectively inhibits phosphodiesterase type 5 (PDE5), the enzyme responsible for breaking down cGMP in vascular smooth muscle cells. By blocking PDE5, sildenafil effectively prolongs cGMP activity, amplifying the vasodilatory response to endogenous nitric oxide. This mechanism underlies its success in treating erectile dysfunction (by improving penile vascular perfusion) and pulmonary arterial hypertension (by reducing pulmonary vascular resistance).
In the context of preeclampsia, sildenafil’s proposed benefits extend across several physiological domains:
- Enhanced Uteroplacental Blood Flow: By promoting vasodilation in the uterine and placental vasculature, sildenafil may counteract the high-resistance state characteristic of preeclampsia.
- Improved Fetal Oxygenation and Growth: Increased perfusion reduces chronic hypoxia, potentially mitigating intrauterine growth restriction (IUGR).
- Endothelial Protection: By restoring cGMP signaling, sildenafil may reduce oxidative stress and inhibit apoptosis in endothelial cells.
- Anti-inflammatory Modulation: Elevated cGMP indirectly suppresses inflammatory cytokine production and leukocyte adhesion to the vascular wall.
Animal models provided striking support for these effects. In rat and sheep models of preeclampsia, sildenafil improved fetal weight, umbilical blood flow, and placental morphology. Additionally, it normalized VEGF/sFlt-1 ratios and reduced placental oxidative markers. These mechanistic findings fueled early enthusiasm that sildenafil might transform preeclampsia from an obstetric emergency to a manageable vascular disorder.
But animal models are mercifully simpler than human pregnancies. What followed in clinical trials revealed the fragile boundary between hope and harm.
Clinical Trials: From Promise to Pause
Early pilot studies in humans were cautiously optimistic. Small open-label trials and case reports suggested that sildenafil improved Doppler indices of uterine and umbilical artery flow, reduced maternal blood pressure, and modestly prolonged pregnancy in severe preeclampsia or IUGR. Some reports noted increased amniotic fluid volume and improved neonatal outcomes when sildenafil was administered late in gestation.
However, as larger randomized controlled trials (RCTs) were launched, the narrative began to shift.
The STRIDER Trials: A Turning Point
The most influential body of evidence came from the STRIDER consortium (Sildenafil Therapy In Dismal prognosis Early-onset intrauterine growth Restriction). Multiple national trials (UK, New Zealand/Australia, and the Netherlands) examined whether sildenafil could improve fetal outcomes in pregnancies complicated by early-onset IUGR — a close physiological cousin of preeclampsia.
The Dutch STRIDER trial was halted prematurely in 2018 due to unexpected neonatal mortality. Infants exposed to sildenafil had a higher incidence of persistent pulmonary hypertension of the newborn (PPHN) — a potentially fatal condition marked by maladaptive pulmonary vasoconstriction. No improvement in fetal growth or survival was observed. The safety signal was alarming enough to suspend other ongoing trials worldwide.
Subsequent analyses across the STRIDER network found no consistent evidence of benefit and, in some cases, possible harm. Meta-analyses confirmed that sildenafil failed to improve perinatal survival, birth weight, or gestational prolongation in preeclamptic and IUGR pregnancies. The adverse pulmonary outcomes in neonates could not be ignored.
What went wrong? The answer lies in the gap between elegant theory and biological reality.
Mechanistic Paradox: When Vasodilation Turns Harmful
The maternal–fetal interface is a uniquely regulated circulatory ecosystem. Sildenafil’s vasodilatory effects, beneficial in systemic or pulmonary vasculature, may behave unpredictably in the placental circulation, which relies on specialized endothelial and trophoblastic signaling.
Three mechanisms have been proposed for the adverse outcomes observed in human trials:
- Disruption of Fetal Pulmonary Transition: Chronic intrauterine exposure to sildenafil may lead to pulmonary vascular remodeling in the fetus. At birth, when pulmonary resistance must fall abruptly to allow air breathing, these vessels may fail to respond appropriately, leading to PPHN.
- Maladaptive Redistribution of Flow: Sildenafil-induced vasodilation may preferentially shunt blood to well-oxygenated areas, paradoxically worsening perfusion in already ischemic placental territories.
- Amplification of Hypoxic Stress: By increasing metabolic demand in compromised fetuses, sildenafil might exacerbate hypoxia in tissues unable to sustain higher perfusion pressures.
These mechanisms highlight a fundamental issue: placental pathology is not simply vasoconstriction. It involves structural maldevelopment, altered receptor expression, and oxidative injury — factors sildenafil cannot reverse. Restoring flow without restoring architecture may be analogous to opening a blocked pipe whose inner lining is disintegrating.
Maternal Outcomes: Some Relief, No Revolution
While fetal results were disappointing, maternal hemodynamics occasionally showed mild improvement. Some studies noted reduced uterine artery resistance and modest blood pressure lowering, though not to clinically meaningful extents. Importantly, sildenafil did not prevent the progression of preeclampsia to severe disease or eclampsia.
From a pharmacological standpoint, maternal benefits were constrained by pharmacokinetics: sildenafil’s half-life and placental transfer rate limit sustained therapeutic concentrations in target tissues. Moreover, variability in PDE5 expression across vascular beds further complicated predictability.
Thus, while the maternal endothelium may respond to cGMP restoration, the placenta — the real culprit — remains largely unaffected.
Lessons Learned: Translational Limitations in Obstetric Pharmacology
The rise and retreat of sildenafil in preeclampsia exemplify the translational gap between preclinical success and clinical safety. Several lessons emerge:
- Species-Specific Placental Biology: Rodent and sheep models possess fundamentally different placental structures and PDE5 expression patterns compared to humans. Drugs that normalize perfusion in one species may disrupt developmental signaling in another.
- Complexity of Fetal–Maternal Pharmacokinetics: The placenta is not merely a passive filter; it is an active metabolic organ. Drug transfer, fetal metabolism, and cumulative exposure vary widely across gestational ages.
- Endothelial Restoration Is Not Placental Regeneration: Sildenafil may improve endothelial tone but cannot correct trophoblast invasion failure or villous malformation — the architectural defects central to preeclampsia.
- Safety Must Trump Enthusiasm: When interventions shift the burden of pathology from mother to fetus, enthusiasm must yield to prudence.
Ultimately, the STRIDER experience underscored a sobering reality: not every rational therapy survives its first encounter with human pregnancy.
Re-examining the Biological Rationale: Is There Still a Role?
Despite the setback, the story of sildenafil in obstetrics is not over. Some researchers argue that the mechanistic rationale remains biologically sound, but the clinical application was mistimed. Perhaps the failure lies not in the concept but in its execution.
Earlier Intervention Hypothesis
One proposition is that sildenafil might be beneficial if administered before severe placental injury occurs — perhaps during the preclinical phase of high-risk pregnancies identified by biomarker screening (e.g., low PlGF or high sFlt-1). Early restoration of uteroplacental flow could, in theory, prevent irreversible vascular remodeling. However, ethical and safety barriers to early pharmacologic intervention in pregnancy remain formidable.
Precision Medicine and Dosing
Future studies may explore individualized dosing based on maternal-fetal pharmacogenomics, PDE5 expression, and hemodynamic response. Lower-dose regimens or shorter treatment durations could mitigate fetal pulmonary risks while preserving maternal benefit.
Alternative PDE5 Inhibitors
Not all PDE5 inhibitors are identical. Tadalafil, with its longer half-life and steadier pharmacokinetics, may achieve more stable vascular effects without the peaks and troughs of sildenafil. Preliminary preclinical studies suggest a more favorable safety profile, but clinical data remain limited.
These hypotheses reflect an important truth: scientific enthusiasm should not die with one failed drug but evolve into better-informed inquiry.
Ethical Reflections: The Responsibility of Innovation
The sildenafil–preeclampsia episode also raises profound ethical questions. Pregnancy is a dual-patient condition, and interventions that may help the mother can inadvertently harm the fetus. The Dutch STRIDER outcome, where a therapy intended to save growth-restricted babies may have contributed to their deaths, underscores the moral weight of experimental obstetric pharmacology.
Future trials must therefore adhere to principles of transparency, interim safety review, and strict risk–benefit justification. Enthusiasm in translational science is valuable, but it must be tempered by humility toward biology’s complexity and the ethical primacy of “first, do no harm.”
Beyond Sildenafil: The Search for Targeted Therapies
The failure of sildenafil does not invalidate the vascular hypothesis of preeclampsia — it merely redirects it. Research has pivoted toward more specific modulators of endothelial and placental function:
- Recombinant VEGF and PlGF analogues to counteract sFlt-1 excess.
- sFlt-1 apheresis or neutralizing antibodies to directly reduce antiangiogenic burden.
- Nitric oxide donors and L-arginine supplementation as physiological precursors to the NO–cGMP pathway.
- Statins (pravastatin), which exhibit pleiotropic endothelial protective effects, currently under clinical evaluation.
Each of these approaches embodies lessons learned from the sildenafil journey: restore vascular health not by brute-force vasodilation, but by correcting molecular imbalances at their root.
Conclusion
The tale of sildenafil in preeclampsia is one of brilliant hypothesis, disappointing translation, and enduring scientific humility. Mechanistically, the drug aligns perfectly with the known pathophysiology of the disorder — endothelial dysfunction, NO deficiency, and placental ischemia. Preclinically, it worked. Clinically, it faltered.
But science progresses as much through its missteps as through its triumphs. The experience has refined our understanding of placental pharmacology, maternal–fetal hemodynamics, and the limits of therapeutic optimism. Sildenafil may not be the silver bullet once hoped for, but its journey reshaped how we design, test, and ethically evaluate drugs in pregnancy.
So, should we still be enthusiastic? Yes — but cautiously, rationally, and with deeper respect for the complexity of the maternal–fetal unit. The enthusiasm now belongs not to sildenafil itself, but to the pursuit of smarter, safer vascular therapies that may one day make preeclampsia a manageable relic of obstetric history.
FAQ: Sildenafil and Preeclampsia
1. Why was sildenafil initially considered for preeclampsia?
Because it enhances nitric oxide–mediated vasodilation through PDE5 inhibition, improving vascular relaxation and potentially increasing uteroplacental blood flow. Preclinical studies in animals showed improved fetal growth and placental function, prompting early clinical enthusiasm.
2. Why did clinical trials show adverse outcomes instead of benefits?
Human placental physiology differs from animal models. Chronic intrauterine sildenafil exposure may disrupt fetal pulmonary vascular development, leading to persistent pulmonary hypertension of the newborn (PPHN). Additionally, it failed to correct the structural placental abnormalities underlying preeclampsia.
3. Is sildenafil completely contraindicated in pregnancy?
It is not approved for routine use in preeclampsia or IUGR outside of clinical trials. However, it remains indicated for pulmonary hypertension during pregnancy under specialist supervision, where maternal benefits outweigh potential fetal risks.