Introduction
In surgery, the closure of wounds inside the body represents a masterpiece of biological and surgical precision. Yet, when that closure involves reconnecting two ends of the colon—a process known as colon anastomosis—the outcome can determine life or death. The complication that haunts surgeons most in this context is the anastomotic leak (AL), where the surgical join fails and intestinal contents escape into the abdomen. This complication occurs in 3–20% of patients and remains one of the leading causes of postoperative morbidity, prolonged hospitalization, and even mortality after colorectal surgery.
The quest to improve anastomotic healing has led researchers to explore innovative solutions that bridge classical surgery and regenerative medicine. Among the most promising are adipose tissue-derived mesenchymal stem cells (AT-MSCs)—versatile cells capable of orchestrating tissue repair—and sildenafil citrate, a well-known phosphodiesterase-5 inhibitor better recognized for enhancing blood flow in other regions of the body. Could these two therapeutic tools, separately or together, restore the delicate balance between inflammation, regeneration, and perfusion at the anastomotic site?
A 2022 experimental study from Aydın Adnan Menderes University in Türkiye addressed precisely this question. Using a Wistar rat model, researchers investigated how AT-MSCs and sildenafil—alone or in combination—affect the complex cascade of wound healing, collagen formation, and inflammation in colon anastomosis. Their results offer fascinating insights into how modern cellular therapy and an old pharmacological ally might converge to strengthen one of surgery’s weakest links.
Understanding the Problem: Why Colon Anastomosis Fails
Anastomotic failure is not merely a technical mishap—it is a biological failure of healing. The process of intestinal repair depends on a finely tuned sequence of overlapping phases:
- Inflammation, where neutrophils and macrophages flood the site to clear debris and pathogens.
- Proliferation, marked by fibroblast migration, angiogenesis, and collagen deposition.
- Remodeling, where newly formed tissue matures and strengthens.
Any interruption—insufficient blood supply, excessive inflammation, poor collagen quality—can derail the process and lead to leakage. Inadequate oxygenation and perfusion are particularly devastating, since the colon is naturally more ischemic than other gastrointestinal segments. Add infection, tissue stress, or systemic illness, and the healing environment becomes hostile.
For decades, surgeons have sought to enhance anastomotic healing using growth factors, hyperbaric oxygen, and biomaterials. However, results have been inconsistent. The focus has now shifted toward biological therapies that can directly influence the cellular microenvironment, modulate inflammation, and promote regeneration at a molecular level.
Adipose-Derived Mesenchymal Stem Cells: Nature’s Reparative Architects
Mesenchymal stem cells (MSCs) derived from adipose tissue are not mere spectators in wound healing—they are active conductors of tissue repair. Harvested from fat, these cells can differentiate into fibroblasts, endothelial cells, and smooth muscle cells, while releasing a symphony of cytokines, growth factors, and extracellular vesicles that reduce inflammation and stimulate tissue regeneration.
Unlike embryonic stem cells, AT-MSCs are ethically acceptable and relatively easy to obtain. Their advantages include:
- High yield and accessibility from adipose tissue.
- Low immunogenicity, allowing for autologous or even allogenic applications.
- Potent paracrine effects, especially in modulating immune responses and collagen synthesis.
Previous studies have shown that AT-MSCs improve healing in cardiac, esophageal, and skin tissues. However, their role in colorectal anastomosis has remained controversial. Earlier trials using different delivery methods—biosutures, cell sheets, serosal injections—produced inconsistent outcomes. The present study sought to clarify their impact under controlled experimental conditions.
Sildenafil Citrate: From Vasodilation to Wound Restoration
Sildenafil citrate, commonly associated with erectile dysfunction treatment, acts as a phosphodiesterase-5 (PDE5) inhibitor, increasing levels of cyclic guanosine monophosphate (cGMP) and thereby enhancing nitric oxide–mediated vasodilation. In simple terms, it improves blood flow, an essential ingredient for wound repair.
Beyond perfusion, sildenafil exhibits anti-inflammatory and antioxidant properties. It has been shown to reduce neutrophil infiltration, promote angiogenesis, and accelerate tissue regeneration in various models of ischemic injury. These properties make it a logical candidate for improving anastomotic healing, where blood supply and inflammation balance are critical.
Yet, prior research on sildenafil’s efficacy in colorectal surgery has been mixed. Some animal studies demonstrated enhanced collagen deposition and reduced adhesions; others reported negligible impact on leak rates or tensile strength. This uncertainty set the stage for combining sildenafil with AT-MSCs—to determine whether improved perfusion could amplify stem cell–mediated regeneration.
The Experimental Design: Four Groups, One Goal
The researchers conducted a meticulously structured experiment using 40 female Wistar rats, divided into four equal groups:
- Control group: Standard colon anastomosis without treatment.
- Sildenafil group: Received 10 mg/kg sildenafil citrate via oral gavage.
- Stem cell group: Received local AT-MSC injections at the anastomotic site.
- Combination group: Received both AT-MSC injections and sildenafil administration.
All animals underwent end-to-end anastomosis of the descending colon, followed by a five-day recovery period. The team evaluated healing through both macroscopic and microscopic assessments, including:
- Burst pressure (mechanical strength of the anastomosis).
- Hydroxyproline content (collagen indicator).
- TNF-α concentration (inflammatory marker).
- Histopathological analysis, focusing on necrosis, leukocyte infiltration, and epithelial regeneration.
- Macrophage density, measured using Iba1 immunohistochemical staining.
By combining biochemical, histological, and mechanical measurements, the study achieved a comprehensive evaluation of wound healing dynamics.
Results: Stem Cells Take the Lead, Sildenafil Supports the Act
The study produced several compelling findings, some predictable, others surprising.
Reduced Adhesions, Stable Mechanical Strength
All treated groups—sildenafil, stem cell, and combination—showed significant reductions in perianastomotic adhesions compared to controls. This suggests a favorable anti-inflammatory environment. However, burst pressure, the classic measure of mechanical integrity, did not differ significantly between groups. This finding mirrors previous reports that collagen quantity alone does not dictate tensile strength; instead, the organization and cross-linking of collagen fibers are what matter most.
Biochemical Improvements: Collagen Up, Inflammation Down
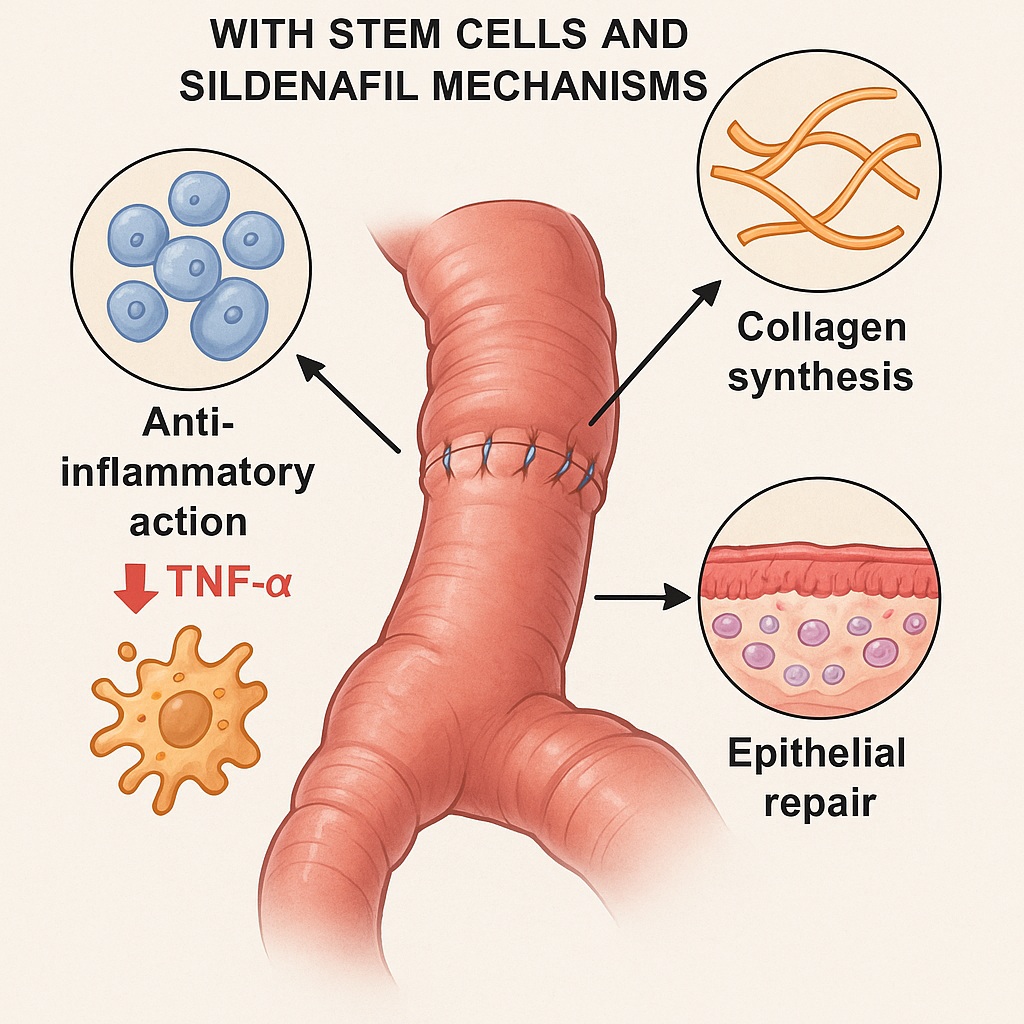
The hydroxyproline content—a reliable indicator of collagen deposition—was markedly higher in both the stem cell and combination groups, reflecting enhanced fibroblast activity and tissue remodeling. Meanwhile, TNF-α levels, a pro-inflammatory cytokine, were significantly lower in those same groups. In contrast, sildenafil alone did not significantly reduce TNF-α, suggesting its anti-inflammatory effects are more indirect, perhaps mediated through improved perfusion rather than cytokine suppression.
Histology: The Microscopic Story
Histopathological analysis provided a vivid picture of tissue recovery:
- The control group showed persistent necrosis, dense neutrophil infiltration, and incomplete epithelial coverage.
- The sildenafil group exhibited modest improvement, with reduced necrosis and a thin epithelial layer beginning to cover granulation tissue.
- The stem cell group achieved nearly complete epithelial regeneration, minimal necrosis, and narrow gaps between wound edges.
- The combination group, though improved, did not surpass the stem cell group alone in overall healing quality.
Macrophage Dynamics: The Inflammatory Modulators
Macrophage density decreased significantly in both the sildenafil and stem cell monotherapy groups but paradoxically increased in the combination group. The researchers speculated that this may represent an increase in anti-inflammatory M2 macrophages, which are known to promote tissue remodeling and angiogenesis. However, since the staining method (Iba1) did not distinguish between M1 and M2 phenotypes, this hypothesis requires further confirmation.
Interpreting the Findings: The Science Behind the Healing
The Role of AT-MSCs in Modulating Inflammation
Stem cells act less like bricklayers and more like conductors of cellular orchestration. They do not necessarily integrate into the regenerating tissue; rather, they secrete signaling molecules that reprogram local immune and stromal cells. In this study, AT-MSCs clearly downregulated TNF-α, suggesting active suppression of inflammatory cascades. This aligns with other research showing that MSCs induce macrophage polarization toward an M2 phenotype, characterized by tissue repair and angiogenesis rather than inflammation.
Sildenafil as a Perfusion Enhancer and Cytokine Modulator
Sildenafil’s primary benefit lies in improving tissue oxygenation and vascular supply, critical for the energy-intensive process of collagen synthesis and epithelial growth. Its modest impact on TNF-α may reflect an indirect mechanism: by reducing ischemic stress, sildenafil prevents excessive cytokine release without directly interfering with immune signaling. It also stimulates nitric oxide pathways that favor angiogenesis and epithelial proliferation.
Why the Combination Didn’t Outperform Stem Cells Alone
Interestingly, the combination therapy did not yield synergistic effects. While this might seem counterintuitive, the explanation likely lies in timing and biological saturation. Stem cells already maximize anti-inflammatory and regenerative signaling; sildenafil’s added perfusion benefit may not significantly enhance this effect within a short five-day observation window. Alternatively, interaction between pharmacological and cellular pathways could alter macrophage behavior in unpredictable ways.
Clinical Relevance: Translating Rat Data to Human Surgery
Although animal models can never fully replicate human physiology, the implications of this study are clinically significant. Colorectal surgery remains a domain where even small improvements in healing can dramatically reduce morbidity. The key takeaways for translational medicine are:
- Stem cell therapy could emerge as a targeted adjunct for high-risk anastomoses, particularly in ischemic or irradiated bowel.
- Sildenafil may be valuable as a perioperative perfusion enhancer, especially in patients with vascular compromise.
- Combination strategies require optimization of dose, timing, and delivery to achieve true synergy.
Future clinical trials might explore localized delivery systems, such as stem-cell–loaded hydrogels or sildenafil-releasing biomaterials, to achieve sustained therapeutic concentrations at the anastomotic site.
The Biology of Healing: From Inflammation to Regeneration
Wound healing in the colon is not a linear progression but a dynamic conversation among immune, stromal, and epithelial cells. This study elegantly demonstrated how altering the conversation can change outcomes.
- Inflammation Phase: Stem cells suppressed excessive TNF-α and neutrophil infiltration, preventing collateral tissue damage.
- Proliferative Phase: Increased hydroxyproline confirmed fibroblast activation and collagen synthesis.
- Regenerative Phase: Enhanced epithelial coverage restored barrier function, reducing the risk of leakage and infection.
Thus, AT-MSCs effectively shortened the inflammatory phase while accelerating the transition to proliferation and regeneration—a therapeutic balance that synthetic drugs rarely achieve.
Limitations and Future Directions
Every study raises as many questions as it answers. The researchers acknowledged several limitations:
- The observation period was limited to five days, capturing only early healing. Longer-term studies are needed to assess collagen maturation and mechanical strength.
- Macrophage subtypes were not differentiated; future work should employ markers distinguishing M1 and M2 populations.
- Dosing and timing of both AT-MSCs and sildenafil may significantly influence outcomes and deserve further optimization.
Additionally, the complex interaction between sildenafil’s nitric oxide pathway and stem cell paracrine signaling warrants molecular-level investigation. Future studies might also consider 3D scaffolds or bioengineered matrices to improve stem cell retention and local action.
Conclusion
The integration of regenerative medicine and pharmacologic modulation represents the next frontier in surgical healing. This experimental study demonstrated that adipose tissue-derived mesenchymal stem cells (AT-MSCs) profoundly enhance early-stage anastomotic repair in the colon by reducing inflammation, promoting collagen synthesis, and stimulating epithelial regeneration. Sildenafil citrate, though less potent alone, contributed to reducing adhesions and modulating the inflammatory milieu.
The most striking conclusion, however, was that stem cells alone achieved the most pronounced improvement—a reminder that sometimes biology’s natural engineers need no assistance. Still, sildenafil remains an attractive partner, potentially useful in clinical contexts where perfusion deficits hinder healing.
Together, these findings illuminate a path toward more biologically intelligent surgical recovery, where the operating room meets the regenerative laboratory. The colon, long considered a reluctant healer, may soon benefit from therapies that truly understand its language of repair.
FAQ: Stem Cells, Sildenafil, and Colon Healing
1. How do adipose-derived stem cells improve colon anastomosis healing?
AT-MSCs release anti-inflammatory and growth-promoting molecules that reduce TNF-α, attract reparative macrophages, stimulate fibroblast activity, and enhance epithelial regeneration—all of which accelerate wound closure and strengthen tissue integrity.
2. Why use sildenafil in a colon healing study?
Sildenafil improves microcirculation and oxygen delivery, both vital for collagen synthesis and cellular energy metabolism. It also exhibits mild anti-inflammatory effects by modulating nitric oxide pathways, helping maintain a favorable healing environment.
3. Can these findings be applied to human surgery?
Not yet directly, but the results are promising. Before clinical use, further research must confirm safety, optimal dosing, and delivery methods in humans. Still, stem cell–based therapies are moving rapidly toward real-world surgical applications.