Introduction: When Modern Medicine Meets Ancient Roots
Sexual health, though often framed in physiological terms, is deeply woven into human emotion and identity. The ability to perform sexually is not merely mechanical—it represents confidence, connection, and vitality. Thus, when sexual dysfunction arises, it reverberates through a person’s entire psychological and relational fabric.
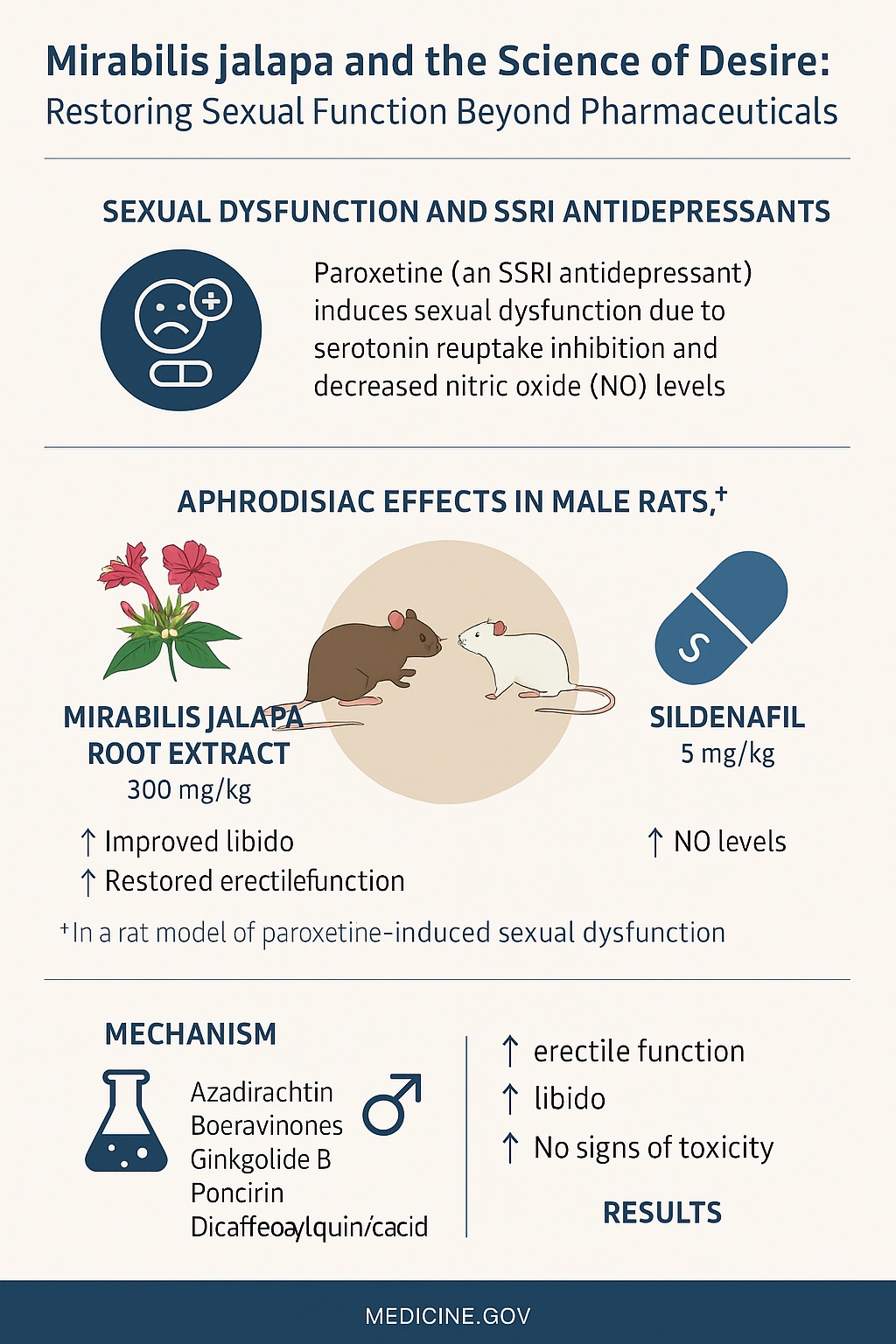
Among the modern culprits, selective serotonin reuptake inhibitors (SSRIs)—particularly paroxetine—stand out. They are effective antidepressants, yet their dark side is well-known: sexual dysfunction. Reduced libido, delayed orgasm, and impaired erection are distressing for patients and clinicians alike. Ironically, the medication that restores emotional balance often suppresses sexual vitality.
In a recent experimental study, researchers turned to a plant that has long been a part of traditional medicine: Mirabilis jalapa L., commonly known as the “four o’clock flower.” Its roots have been reputed to boost male virility in various folk systems. In a rigorously designed animal model, investigators compared its aphrodisiac potential with that of sildenafil, the gold-standard pharmaceutical for erectile dysfunction. The results reveal a fascinating convergence of botany and biochemistry, suggesting that ancient remedies may hold keys to modern problems.
The Paroxetine Problem: When Serotonin Silences Desire
SSRIs revolutionized psychiatry by making depression treatment more tolerable and accessible. Yet, they come with a paradoxical cost. By increasing synaptic serotonin, they improve mood but dampen sexual arousal through inhibition of nitric oxide synthase and reduction of testosterone and gonadotropins.
In clinical practice, this means that many patients trade sadness for sexual indifference. Studies estimate that up to 70% of SSRI users experience some degree of sexual dysfunction. For men, paroxetine is the most notorious offender. It delays ejaculation, reduces penile rigidity, and can even induce temporary impotence.
Animal studies mirror this pattern. Male rats exposed to paroxetine show diminished mount frequency, lower testosterone, and longer post-ejaculatory intervals—biological reflections of a frustrated libido. It is a model that mimics human SSRI-induced sexual dysfunction with uncanny accuracy, providing a platform to test potential treatments.
Mirabilis jalapa: The Four O’Clock Flower with Timeless Secrets
Mirabilis jalapa, native to tropical America but now cultivated worldwide, is best known for its colorful evening blooms. Traditional healers, however, have long valued its roots for their rejuvenating and aphrodisiac properties. In Ayurvedic and folk Pakistani medicine, powdered roots are taken to enhance male potency, relieve fatigue, and restore sexual vigor.
The plant’s pharmacological interest extends far beyond tradition. Modern phytochemistry has identified multiple classes of compounds within its tissues: flavonoids, rotenoids, alkaloids, and phenolic acids, each with potential bioactivity. Yet until recently, little was known about its specific effects on antidepressant-induced sexual dysfunction.
This gap inspired the 2023 study by Rahman and colleagues, who set out to scientifically validate Mirabilis jalapa’s reputation and explore its biochemical composition using advanced UPLC-MS (ultra-high-performance liquid chromatography–mass spectrometry).
Study Design: A Scientific Romance Between Plants and Pharmacology
The study employed male Wistar rats, a standard model for behavioral and pharmacologic experiments. Sexual depression was induced by chronic paroxetine administration (20 mg/kg) over 21 days. The animals were then divided into five groups:
- Control: distilled water only
- Paroxetine: SSRI-treated, no intervention
- Sildenafil + Paroxetine: 5 mg/kg sildenafil co-treatment
- Mirabilis extract + Paroxetine: 300 mg/kg root extract
- Mirabilis extract alone: 300 mg/kg extract without paroxetine
Sexual behavior was recorded during nightly pairing sessions with hormonally primed female rats. Researchers quantified classic sexual parameters—mount frequency, intromission frequency, ejaculation latency, and post-ejaculatory interval—using video analysis.
Meanwhile, the extract’s chemical fingerprint was analyzed via UPLC-MS-TOF, revealing the molecular identities of the active compounds.
Results: When the Flower Outperformed the Pill
The results were both striking and consistent. Mirabilis jalapa extract at 300 mg/kg significantly enhanced sexual activity across multiple parameters:
- Mount and intromission frequencies increased steadily throughout the 21-day period, approaching or exceeding those observed with sildenafil.
- Ejaculatory frequency—a key indicator of libido and erection quality—rose markedly in treated rats compared to paroxetine-only controls.
- Mount and intromission latencies decreased, suggesting heightened sexual motivation and faster initiation of mating.
- Post-ejaculatory intervals were shorter, reflecting quicker recovery and sustained desire.
In essence, the extract reversed the depressive effects of paroxetine on male sexual behavior, restoring natural patterns of arousal and performance.
What’s more, the combination of Mirabilis extract with paroxetine performed comparably to sildenafil co-treatment, indicating that the herbal compound can counteract SSRI-induced dysfunction without major adverse effects.
Phytochemical Insights: Nature’s Molecular Pharmacology
The UPLC-MS analysis uncovered a complex cocktail of bioactive phytoconstituents, many reported here for the first time in Mirabilis jalapa roots. Among them:
- Ginkgolide B – a terpene lactone known to enhance cerebral and penile blood flow.
- Boeravinone C and F – rotenoids with antioxidant and genoprotective properties.
- Poncirin – a flavanone glycoside with anti-inflammatory and smooth-muscle-relaxing effects.
- 1,5-Dicaffeoylquinic acid – a neuroprotective polyphenol that modulates nitric oxide signaling.
- Azadirachtin – a triterpenoid previously known for hepatoprotective activity, newly identified here.
- Chrysophanol – an anthraquinone with antimicrobial and anti-inflammatory properties.
- Cyanidin-3-O-galactoside – an anthocyanin with strong antioxidant capacity.
Together, these compounds form a phytochemical symphony targeting oxidative stress, vascular flow, and endocrine modulation—the very pathways disrupted by SSRIs.
Interestingly, ginkgolide-type molecules enhance nitric oxide (NO) availability, a key mediator of penile erection. The presence of such compounds helps explain why Mirabilis extract not only improved libido but also sustained erection quality in paroxetine-treated rats.
Mechanistic Pathways: Restoring the NO Symphony
Sexual arousal and erection depend on a finely tuned cascade of neurovascular signals. Nitric oxide, produced by nitric oxide synthase (NOS), triggers cyclic GMP accumulation in smooth muscle, leading to vasodilation and penile rigidity.
Paroxetine disrupts this process by inhibiting NOS, reducing NO synthesis, and lowering testosterone levels. Mirabilis jalapa appears to restore this cascade through multiple mechanisms:
- Enhancing NO bioavailability via flavonoids and phenolic acids that stimulate endothelial NOS.
- Reducing oxidative stress, thereby protecting NO from degradation by reactive oxygen species.
- Balancing hormonal profiles, possibly through steroid-like saponins that normalize testosterone.
The cumulative effect is a revival of sexual reflexes, observable in increased mount and intromission rates. In other words, Mirabilis doesn’t simply mimic sildenafil—it rebuilds the biochemical terrain that allows arousal to flourish.
Behavioral Outcomes: A Lesson in Libido and Motivation
In sexual behavior research, numbers are only part of the story. The qualitative observation of rat behavior reveals subtler truths about motivation and arousal. Paroxetine-treated males were sluggish, hesitant, and often disengaged from female cues. Those given Mirabilis extract displayed restored pre-copulatory behaviors—sniffing, pursuit, and mounting—with vigor and persistence.
These findings suggest not only physiological recovery but also psychosexual reactivation. The extract’s influence on dopamine-serotonin balance may underlie this behavioral renewal, enhancing desire alongside performance.
Interestingly, the combination of Mirabilis and paroxetine did not produce hypersexual behavior or toxicity, indicating a therapeutic balance rather than overstimulation—a desirable trait for any potential aphrodisiac.
Comparing Nature and Pharmacology: A Subtle Rivalry
Sildenafil, the widely marketed PDE5 inhibitor, remains the benchmark for treating erectile dysfunction. However, its mechanism is purely vascular—it enhances blood flow but does not influence desire or mood. Moreover, it may not correct SSRI-induced dysfunction, which stems from central serotonergic inhibition rather than local vasculature.
Mirabilis jalapa, on the other hand, operates on multiple fronts: it modulates neurotransmitters, supports hormonal balance, improves blood flow, and reduces oxidative damage. In essence, it acts as a multi-target adaptogen rather than a single-target drug.
This doesn’t mean it will replace sildenafil, but rather complement it. Where sildenafil pushes physiology, Mirabilis restores it.
Safety and Ethical Perspectives
Toxicological signs were notably absent in this study. The rats maintained normal feeding, grooming, and activity patterns throughout treatment. This safety profile supports traditional observations of Mirabilis as a well-tolerated medicinal root.
However, translating animal data to human application requires caution. Herbal extracts vary in concentration and composition depending on soil, season, and extraction method. Rigorous dose standardization and clinical trials are essential before recommending Mirabilis as an adjunct to SSRI therapy.
Ethically, the integration of traditional knowledge with modern pharmacology underscores the importance of biocultural respect. Plants like Mirabilis jalapa carry centuries of empirical wisdom; scientific validation should honor, not exploit, those traditions.
Phytoconstituents and Future Drug Discovery
Perhaps the most exciting aspect of this research lies not in the behavioral outcomes but in the molecular discoveries. The UPLC-MS fingerprint of Mirabilis jalapa roots revealed over 20 bioactive compounds, several newly reported in this species. These molecules represent a treasure map for pharmaceutical innovation.
For instance:
- Boeravinones and mirabijalones, unique rotenoid structures, exhibit antioxidant and neuroprotective effects—potentially valuable for treating neurovascular disorders beyond sexual dysfunction.
- 1,5-Dicaffeoylquinic acid shows HIV-1 inhibition and neuroprotection, expanding the plant’s medicinal relevance.
- Poncirin and chrysophanol possess anti-inflammatory and antimicrobial properties, which could synergize with sexual health by improving systemic vascular function.
In other words, the aphrodisiac effect may be just the tip of the phytochemical iceberg.
Discussion: When Tradition Anticipates Science
From a broader perspective, this study exemplifies how traditional medicine often predicts pharmacology by centuries. Healers who prescribed Mirabilis for “loss of vigor” were not operating on mysticism but on keen empirical observation. They may not have known about nitric oxide synthase or dopamine modulation, but they observed outcomes—exactly the kind modern science now quantifies.
Moreover, this line of research bridges two worlds too often kept apart: psychopharmacology and ethnobotany. The former seeks molecular precision; the latter, holistic restoration. Mirabilis jalapa sits comfortably between the two, offering biochemical depth with natural balance.
If future studies confirm similar effects in humans, Mirabilis could become a plant-based adjunct for men experiencing SSRI-related sexual suppression—a natural ally to both psychiatry and urology.
Conclusion: Rediscovering Desire Through the Lens of Science
The story of Mirabilis jalapa is a reminder that medicine’s evolution is not always linear. While paroxetine and sildenafil represent the pinnacle of synthetic pharmacology, this unassuming tropical flower reveals that nature still holds the blueprints of balance.
By restoring nitric oxide pathways, enhancing hormonal harmony, and rekindling sexual motivation, Mirabilis jalapa root extract proved capable of reversing antidepressant-induced sexual dysfunction in male rats. Its diverse phytochemical profile—rich in ginkgolides, flavonoids, and phenolic acids—supports a multifaceted mechanism combining vascular, neurological, and endocrine modulation.
In the end, this research is more than a validation of folklore—it is an invitation to rethink how we approach sexual health: not through isolated targets but through systemic restoration. In a world obsessed with quick fixes, perhaps the real aphrodisiac is patience, persistence, and a return to the natural intelligence of plants.
FAQ: Understanding Mirabilis jalapa and Sexual Health
1. Can Mirabilis jalapa be used as a natural alternative to Viagra?
Not directly. While it shows comparable effects in animal models, human studies are lacking. However, it may serve as a safer, multi-target supplement supporting libido, erection quality, and hormonal balance—especially in men affected by antidepressant therapy.
2. How does Mirabilis jalapa differ from standard SSRIs or PDE5 inhibitors?
Unlike pharmaceuticals that act on single pathways, Mirabilis affects multiple biological systems: serotonin modulation, nitric oxide release, vascular tone, and antioxidant defense. This integrated effect makes it potentially useful for complex conditions like SSRI-induced sexual dysfunction.
3. Is it safe for human use?
Traditional use suggests good tolerability, and animal studies reported no toxicity. Nevertheless, standardized extracts and controlled clinical trials are needed to confirm human safety and optimal dosing. Until then, it should be viewed as an experimental herbal candidate, not a certified medication.