Introduction
In modern pharmaceutics, the challenge is rarely about discovering new molecules—it is about making old molecules perform better. Sildenafil citrate, known globally as the active ingredient in Viagra, stands as a case in point. Despite its pharmacologic brilliance as a selective phosphodiesterase type 5 (PDE5) inhibitor, sildenafil has long been hampered by two fundamental physicochemical limitations: poor solubility and delayed absorption.
For a compound intended to deliver swift hemodynamic and erectile responses, these barriers are not trivial inconveniences—they are therapeutic handicaps. The traditional oral tablet form achieves only about 40% bioavailability, with peak plasma concentration delayed by nearly an hour. This delay undermines spontaneity, compliance, and ultimately, patient satisfaction.
A team of Thai pharmaceutical scientists from Walailak and Prince of Songkla Universities proposed an elegant, deceptively simple solution: the dry foam tablet. Using this novel formulation strategy, they transformed sildenafil citrate into a rapidly dissolving, highly porous, and amorphous matrix that releases its entire drug content within five minutes, regardless of pH environment.
Beyond the remarkable numbers lies a larger story—one that speaks to the evolution of drug delivery science: from optimizing dissolution kinetics to reimagining how dosage form engineering can shape the pharmacologic experience itself.
The Problem with Conventional Sildenafil Tablets
Every effective therapy must navigate the biology of absorption, distribution, metabolism, and excretion (ADME). Sildenafil’s journey through this process is less than ideal. It belongs to Biopharmaceutics Classification System (BCS) Class II, meaning it has high permeability but low solubility. While this allows for ready membrane diffusion, dissolution in gastrointestinal fluids becomes the rate-limiting step.
When administered as a standard tablet, sildenafil citrate faces multiple hurdles:
- Limited aqueous solubility (~4 mg/mL), leading to incomplete dissolution in the gastric environment.
- Aggregation of hydrophobic drug particles, reducing the surface area available for solvent contact.
- Variable gastric pH among patients, which alters dissolution kinetics and delays absorption onset.
The pharmacokinetic consequences are well-documented. Even when fully absorbed, sildenafil’s oral bioavailability hovers around 38–41%, heavily influenced by food intake and first-pass metabolism. Moreover, the drug’s onset—typically 30–60 minutes post-ingestion—restricts spontaneous use and patient compliance.
For a therapeutic class that depends on both efficacy and timing, these drawbacks underscore a need for formulation-driven innovation, not molecular reinvention.
The Dry Foam Concept: A New Frontier in Solid Dosage Engineering
Dry foam technology, though relatively new, is rapidly gaining traction as a transformative drug delivery platform. At its core lies a simple but powerful principle: improving drug–solvent interaction by creating a porous, readily wettable structure that resists particle agglomeration.
In the sildenafil study, the researchers leveraged this concept through a three-stage process:
- Foam Formation
Sildenafil citrate was first suspended in a mild surfactant solution—2% sodium dodecyl sulfate (SDS)—to enhance wetting and prevent hydrophobic aggregation. A diluent mixture of maltodextrin and mannitol (1:1) was incorporated to create a stable, viscous paste. When passed through a fine spray nozzle, this mixture produced a homogeneous foam with air dispersed uniformly throughout the matrix. - Drying and Granulation
The foam was dehydrated under vacuum conditions, yielding a dry, highly porous structure. Once dried, it was sieved to form granules (No. 18 mesh), which exhibited exceptional flowability and minimal interparticle friction—a crucial factor for consistent tablet compression. - Tableting and Characterization
The dry foam granules were combined with standard excipients (lactose, croscarmellose sodium, magnesium stearate) and compressed into 600 mg tablets equivalent to 100 mg sildenafil. These tablets showed desirable mechanical strength (hardness ~5 kg), low friability (<1%), and rapid disintegration (<5 minutes).
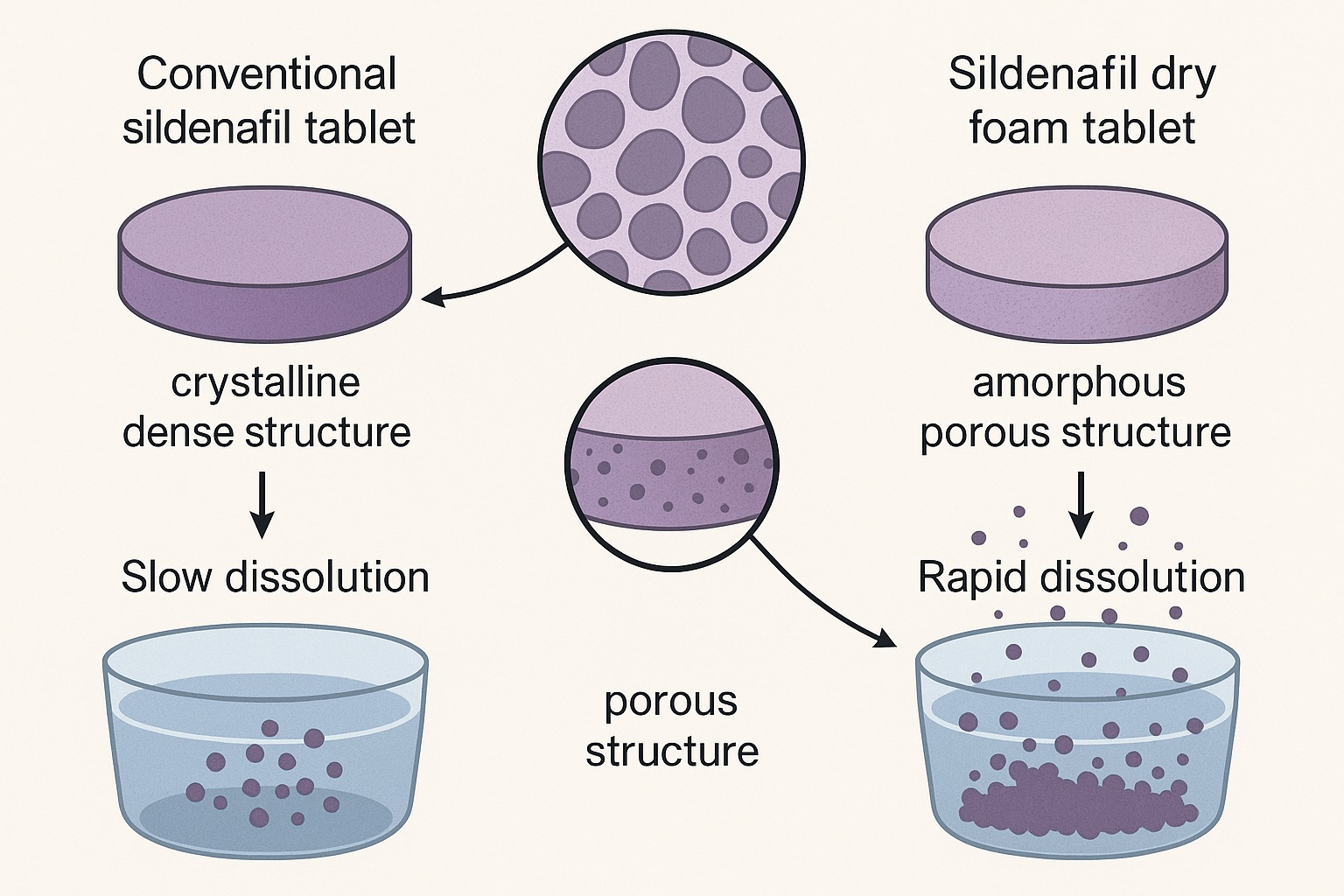
This process fundamentally re-engineered sildenafil’s microstructure—from crystalline to amorphous, from dense to porous, and from hydrophobic to hydrophilic—thereby accelerating its dissolution across physiologic pH conditions.
Mechanistic Insights: Why Porosity Matters
The brilliance of the dry foam tablet lies in its microstructural physics. When observed under scanning electron microscopy (SEM), the granules reveal a labyrinth of microscopic pores—each acting as a capillary conduit that rapidly absorbs aqueous fluid upon contact. This instant hydration prevents particle clumping and allows uniform solvent penetration.
The result is a dissolution profile that borders on the ideal:
- 100% of sildenafil released within 5 minutes, compared to significantly slower rates for conventional tablets.
- Consistent dissolution across acidic, neutral, and mildly basic media (pH 2.0, 4.5, 6.8).
This pH independence is particularly valuable, as gastric acidity varies widely among individuals and is often reduced by concomitant medications such as proton pump inhibitors. The amorphous nature of the drug within the dry foam matrix further contributes to its superior solubility by eliminating the rigid crystal lattice that ordinarily resists hydration.
From a thermodynamic standpoint, amorphous forms possess higher free energy and thus dissolve more readily than their crystalline counterparts. The trade-off—potential instability—is mitigated by the foam’s polymeric carrier system (maltodextrin and mannitol), which stabilizes the amorphous state and prevents recrystallization during storage.
Pharmaceutical Performance: The Numbers That Matter
A formulation’s elegance is only as good as its performance metrics. The sildenafil dry foam tablets demonstrated a constellation of favorable pharmaceutical properties that make them not just an academic novelty, but a commercially viable alternative.
- Flow Properties
The granules exhibited an angle of repose <10°, indicating excellent flowability—a vital factor for uniform die filling during compression. - Tablet Integrity
With a hardness around 5 kg and friability under 1%, the tablets balanced mechanical strength and disintegration speed. - Disintegration and Dissolution
Complete disintegration occurred within five minutes, correlating with near-instantaneous drug release. - Dissolution Consistency
The dissolution curve showed linear, rapid drug release, unaffected by environmental pH—a hallmark of formulation robustness.
In lay terms, these metrics translate to predictable pharmacokinetics, improved onset of action, and greater patient satisfaction—a trio of outcomes that any formulation scientist dreams of achieving.
Translational Implications: From Bench to Bedside
The implications of this work reach far beyond sildenafil. The dry foam method represents a scalable, solvent-free, and cost-effective approach to improving dissolution for BCS Class II drugs, which constitute nearly 40% of the global pharmaceutical market.
For sildenafil itself, the benefits could be transformative. A tablet that dissolves completely in five minutes could shorten onset of action, enabling more spontaneous use—a clinically meaningful outcome for men with erectile dysfunction seeking predictability and discretion. Moreover, enhanced dissolution may allow for dose reduction, minimizing adverse effects such as flushing, headache, or hypotension without sacrificing efficacy.
In the context of pulmonary arterial hypertension (PAH)—another therapeutic domain for sildenafil—faster dissolution could improve hemodynamic control and treatment adherence, particularly in acute or pediatric care settings.
From a manufacturing perspective, dry foam processing avoids solvent-related toxicity and environmental burdens associated with wet granulation, while its reliance on food-grade excipients simplifies regulatory compliance. The process is compatible with continuous manufacturing platforms, aligning with the pharma industry’s shift toward Quality by Design (QbD).
The Broader Scientific Context: The Future of Fast-Dissolving Formulations
The dry foam concept fits into a growing paradigm shift in pharmaceutical design: performance-based formulations that engineer not only drug release but also patient experience. Rapid-dissolving technologies—whether in foam, film, or microgranule form—aim to blur the line between oral solids and liquids, marrying convenience with bioavailability.
For sildenafil and similar drugs, future directions could include:
- Orodispersible foam tablets, enabling sublingual absorption for even faster onset.
- Combination delivery systems, pairing sildenafil with vasodilatory or antioxidant adjuncts.
- Personalized dissolution profiles, achieved through additive manufacturing (3D printing) of foam matrices with tunable porosity.
Dry foam technology’s versatility allows these innovations to materialize with minimal modification of existing production lines—a practical boon in an industry often constrained by infrastructure inertia.
A Critical Perspective: Strengths, Limitations, and Next Steps
Every promising formulation warrants scrutiny. While the dry foam approach offers undeniable advantages, it also raises important questions for future research.
First, the pharmacokinetic equivalence of the foam tablet to standard sildenafil must be established in vivo. Rapid dissolution does not automatically translate to proportionally faster absorption—factors such as intestinal permeability and first-pass metabolism still apply.
Second, stability over shelf life must be verified. Amorphous drugs are notoriously prone to recrystallization under humid conditions, potentially compromising performance. Accelerated stability testing and packaging optimization are essential next steps.
Finally, scale-up and industrial reproducibility remain to be demonstrated. While vacuum drying and sieving are feasible at lab scale, their translation to high-throughput manufacturing requires process validation and cost-benefit analysis.
Nevertheless, these caveats do not diminish the innovation’s potential—they simply chart the course for its evolution from laboratory curiosity to commercial reality.
Conclusion
The development of sildenafil dry foam tablets exemplifies how thoughtful formulation design can breathe new life into well-known drugs. By transforming the molecule’s dissolution kinetics, researchers achieved what might be called pharmaceutical alchemy: turning a reluctant, slow-dissolving solid into a rapidly bioavailable form without altering its chemistry.
The achievement is not merely technical; it is therapeutic. In a field where patient satisfaction depends as much on timing as on efficacy, the ability to shorten onset of action can profoundly enhance quality of life. Moreover, dry foam technology stands as a platform innovation with broad applicability across pharmacologic classes.
In the end, this is more than a story about sildenafil—it is a lesson in pharmaceutical ingenuity. Sometimes, the most profound progress in medicine comes not from discovering new drugs, but from reimagining how old ones dissolve.
FAQ: Sildenafil Dry Foam Tablets
1. How do dry foam tablets differ from conventional tablets in function and design?
Dry foam tablets contain air-infused, porous granules that dissolve almost instantly upon contact with fluid. This structural innovation enhances wettability and prevents drug particle aggregation, resulting in dramatically faster dissolution compared to dense conventional tablets.
2. Will faster dissolution necessarily lead to faster therapeutic onset?
In most cases, yes—but not always. Enhanced dissolution increases the rate of drug absorption, though systemic onset still depends on intestinal permeability and metabolism. Clinical studies are required to confirm the correlation between rapid release and pharmacodynamic onset.
3. Could the dry foam method be applied to other poorly soluble drugs?
Absolutely. The technology is particularly suitable for BCS Class II and IV compounds, where solubility—not permeability—is the limiting factor. It holds promise for drugs in cardiology, oncology, and neurology that require rapid or consistent plasma levels.