Introduction
For decades, reproductive medicine has pursued one elusive goal: transforming a suboptimal endometrium into a receptive one. While in vitro fertilization (IVF) has advanced dramatically, success rates remain stubbornly constrained by one variable—endometrial quality. A thin or poorly vascularized endometrium is among the most intractable barriers to implantation, pregnancy maintenance, and live birth. In this context, the repurposing of sildenafil citrate, better known as Viagra, emerged as both a logical and seductive proposition.
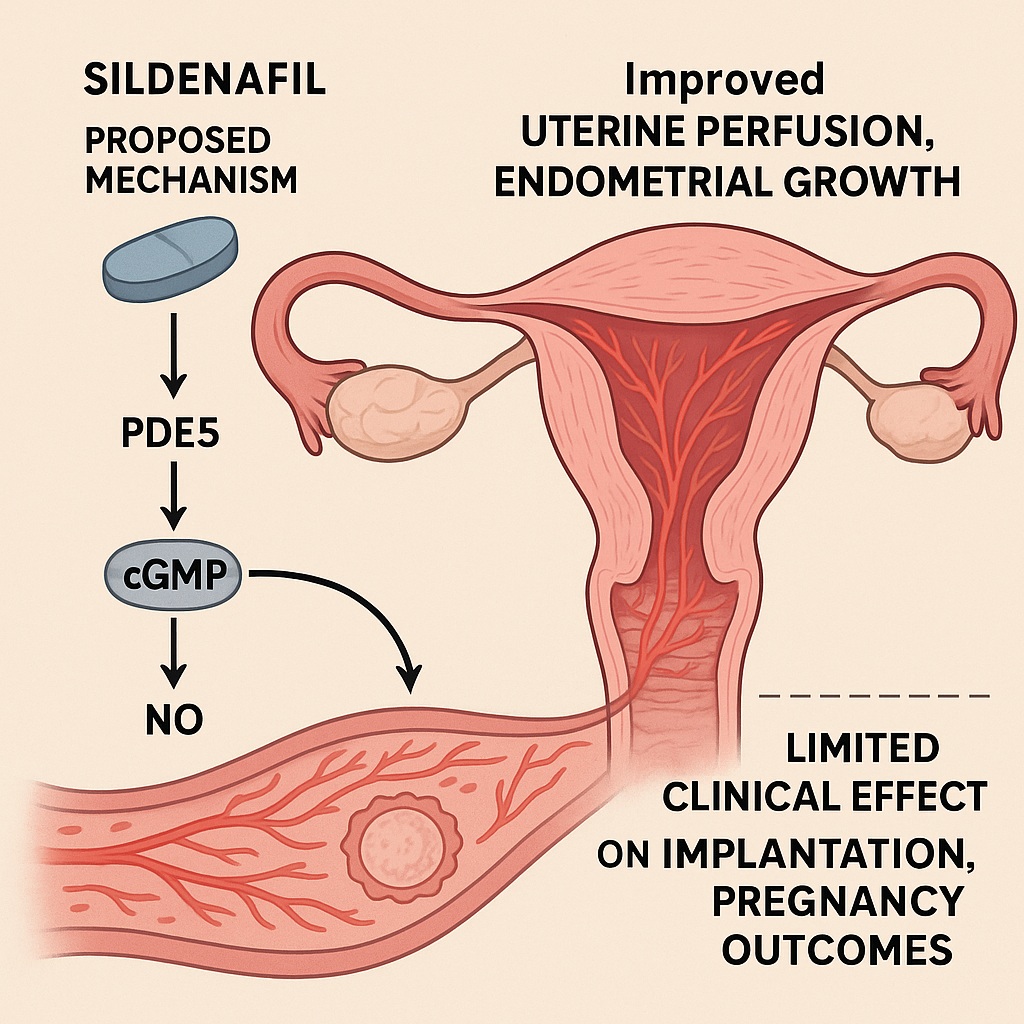
Sildenafil’s well-established pharmacology—phosphodiesterase type 5 (PDE5) inhibition—enhances nitric oxide (NO)–cyclic guanosine monophosphate (cGMP) signaling, promoting vasodilation and increased tissue perfusion. Theoretically, when applied to the uterus, this mechanism should enhance endometrial blood flow, cellular proliferation, and angiogenesis, leading to a thicker and more receptive lining. Early case reports and small pilot studies indeed hinted at improved outcomes, particularly in women with recalcitrant “thin endometrium.”
However, as the latest clinical data from 2022 demonstrate, the story is more complicated. Despite its elegant biochemical rationale, sildenafil has produced inconsistent or negligible clinical benefit in controlled studies. The result is a fascinating paradox: a drug that seems biologically perfect for the problem but clinically underwhelming. Understanding why requires a closer look at both uterine physiology and the nuances of nitric oxide biology.
The Thin Endometrium Problem: A Persistent Challenge in IVF
In the lexicon of reproductive medicine, the “thin endometrium” (TE) represents a formidable obstacle. Clinically, it is defined as an endometrial thickness below 7 mm on the day of embryo transfer, as assessed by transvaginal ultrasound. While numbers alone do not always capture biological receptivity, a thin lining typically correlates with poor vascularity, inadequate stromal development, and reduced expression of adhesion molecules critical for implantation.
The causes of TE are diverse. Asherman’s syndrome, chronic endometritis, prolonged use of clomiphene citrate, radiation, or iatrogenic trauma from curettage can all compromise endometrial regeneration. More insidiously, aging and endothelial dysfunction diminish uterine perfusion, leading to hypoxic tissue that fails to respond even to hormonal priming.
From a physiological standpoint, endometrial receptivity depends on the harmonious interplay of estrogen-driven proliferation and vascular remodeling. The spiral arteries must dilate, the stroma must undergo controlled edema, and the surface epithelium must express a synchronized molecular dialogue with the embryo. Any disturbance—whether vascular, hormonal, or inflammatory—can tip this balance, yielding a thin, poorly perfused endometrium.
Sildenafil, by enhancing nitric oxide–cGMP signaling, seemed poised to correct exactly this imbalance. The hope was not merely to thicken the lining, but to restore its functional receptivity.
The Mechanistic Rationale: From Corpus Cavernosum to Uterus
Sildenafil’s journey from the bedroom to the fertility clinic is, in a sense, a tale of shared physiology. The corpus cavernosum and the uterine endometrium both depend on endothelial nitric oxide release for vascular relaxation. In penile tissue, sildenafil blocks PDE5-mediated cGMP breakdown, sustaining smooth muscle relaxation and blood inflow. Translating this mechanism to the uterus, sildenafil could theoretically increase uterine artery flow, reduce vascular resistance, and enhance nutrient and oxygen delivery to the endometrium.
This improved perfusion should, in turn, promote cellular proliferation, angiogenesis, and glandular development—the very hallmarks of a receptive lining. Sildenafil’s secondary effects, including mild anti-inflammatory and antioxidant activity, may further protect the endometrium from oxidative stress, an underappreciated factor in implantation failure.
Moreover, sildenafil’s influence extends to molecular signaling. NO and cGMP modulate not only vascular tone but also gene expression in stromal and epithelial cells, including the upregulation of vascular endothelial growth factor (VEGF) and endometrial integrins. Theoretically, these molecular cascades converge to create a more hospitable implantation niche.
This convergence of vascular and molecular benefits made sildenafil an irresistible candidate for endometrial rescue. Yet, as the 2022 clinical outcomes make clear, biological plausibility does not always translate into clinical success.
The Study in Focus: Clinical Outcomes of Sildenafil in Thin Endometrium
The 2022 study published in Gynecology and Obstetrics Clinical Medicine investigated sildenafil’s real-world clinical impact in women with poor endometrial development during IVF/ICSI cycles. The retrospective cohort included 472 patients, of whom 88 received vaginal sildenafil (50 mg/day) and 384 served as controls. The primary endpoints were endometrial thickness, morphology, and pregnancy outcomes, including biochemical pregnancy, clinical pregnancy, miscarriage, and live birth rates.
The results were sobering. After adjusting for confounding variables—such as age, infertility duration, and embryo quality—sildenafil did not significantly improve endometrial thickness, morphology, or pregnancy outcomes compared to the control group. While some patients did experience mild increases in thickness, these changes were not statistically or clinically meaningful.
Interestingly, subgroup analyses suggested that sildenafil may benefit a small subset of women—those with severe baseline hypoperfusion or unresponsive endometria—but this effect was inconsistent and unpredictable. The study concluded that, despite its mechanistic appeal, sildenafil’s clinical utility in endometrial enhancement remains unproven.
For clinicians, this raises an uncomfortable but important question: Why does a drug that so elegantly targets the presumed physiological deficit yield such modest results?
When Theory Meets Biology: Why Sildenafil Falls Short
The discrepancy between sildenafil’s theoretical promise and its clinical performance likely arises from the multifactorial nature of endometrial dysfunction. While vascular insufficiency is one contributor, the problem often extends far deeper into cellular, hormonal, and inflammatory domains.
First, endometrial atrophy is not always a perfusion issue. In women with fibrosis, scarring, or basal gland loss (as in Asherman’s syndrome), improving blood flow does not regenerate the structural template for proliferation. Without viable progenitor cells or stromal integrity, sildenafil cannot resurrect what no longer exists.
Second, nitric oxide biology itself is context-dependent. Chronic inflammation, oxidative stress, or estrogen deficiency can impair endothelial NO synthase (eNOS) activity, leading to reduced NO production. Under such conditions, PDE5 inhibition offers little benefit because there is too little NO to amplify. In essence, sildenafil is amplifying silence.
Third, vascular remodeling in the endometrium is hormonally modulated. If estrogen signaling is impaired, the angiogenic response remains blunted regardless of PDE5 activity. Thus, sildenafil’s efficacy may hinge on adequate hormonal priming—a variable not always controlled or optimized in clinical settings.
These insights remind us that sildenafil is not a magic wand for endometrial repair. It is a pharmacologic amplifier of physiological processes that must already be active. When those processes are dormant, the amplifier yields no sound.
The Route of Administration: Vaginal Versus Oral Dosing
Another critical variable in sildenafil’s reproductive use is route of administration. Vaginal delivery offers theoretical advantages: direct uterine absorption, minimal systemic exposure, and higher local bioavailability. However, pharmacokinetic studies reveal that vaginal absorption is inconsistent and subject to individual variation in mucosal permeability and local blood flow.
Oral sildenafil, while more predictable in plasma kinetics, produces systemic vasodilation and potential side effects such as headache or flushing, which may be undesirable in fertility patients. Moreover, oral dosing might not achieve adequate tissue concentrations in the uterus, as the drug undergoes extensive hepatic metabolism.
The 2022 study utilized vaginal administration, yet results remained modest, suggesting that delivery route alone cannot overcome biological limitations. Some investigators have proposed combining sildenafil with estrogen or low-dose aspirin to synergize vascular and hormonal effects, though this approach remains experimental and data are inconclusive.
Sildenafil Beyond Thickness: Exploring Functional Receptivity
A crucial limitation of most sildenafil studies lies in their reliance on endometrial thickness as the primary endpoint. While convenient to measure, thickness is an imperfect surrogate for functional receptivity. Numerous studies have shown that implantation can occur in linings thinner than 7 mm, provided that vascular and molecular conditions are favorable. Conversely, a thick but fibrotic or poorly oxygenated endometrium may remain infertile.
Functional receptivity depends on a complex interplay of gene expression, cytokine balance, and immunologic tolerance—domains that sildenafil does not directly modulate. It is conceivable that sildenafil enhances perfusion without meaningfully altering these deeper layers of receptivity, explaining its disconnect from pregnancy outcomes.
Future research should therefore shift from simple morphometric endpoints to molecular and perfusion-based markers—for instance, Doppler indices of uterine artery resistance, VEGF expression, or endometrial receptivity array (ERA) profiles. Only through such multidimensional assessment can we truly gauge whether sildenafil improves not just thickness, but quality.
Translational Implications: Toward Personalized Uterine Perfusion Therapy
Despite mixed results, sildenafil’s story is far from over. The broader concept it represents—targeted modulation of uterine blood flow and microvascular function—remains deeply relevant to reproductive medicine. The failure of uniform benefit does not negate the possibility of personalized efficacy.
In particular, patients with documented uterine hypoperfusion, as measured by Doppler ultrasound, may still represent a sildenafil-responsive subgroup. Likewise, women with recurrent implantation failure (RIF) or thin endometrium secondary to endothelial dysfunction might benefit when sildenafil is part of a multi-agent regimen including estrogen, antioxidants, and low-dose vasodilators.
Another promising frontier lies in preconditioning the endometrium before frozen embryo transfer (FET). Short-term sildenafil administration may improve oxygenation and angiogenesis during the proliferative phase, creating a more favorable environment for implantation in subsequent cycles.
Ultimately, sildenafil’s role may not be as a standalone therapy, but as an adjunctive pharmacologic tool—one piece in the broader puzzle of uterine rejuvenation.
The Broader Lesson: Rethinking Pharmacologic Receptivity Enhancement
The sildenafil story offers a broader lesson about how medicine approaches complex biological systems. The endometrium is not a passive tissue awaiting perfusion; it is an adaptive organ governed by intricate endocrine, paracrine, and immune signaling. Attempts to “fix” it with single-target drugs often fail because they underestimate its complexity.
A more fruitful strategy may involve systems-level approaches—combining pharmacologic, regenerative, and biological interventions. Emerging research into stem cell therapy, platelet-rich plasma (PRP), and growth factor infusions aims to restore endometrial architecture rather than merely dilate its vessels. In such contexts, sildenafil might serve as a supportive agent, improving perfusion to aid cellular engraftment and regeneration.
Thus, sildenafil’s inconsistent outcomes do not signify irrelevance; they highlight the necessity of integrated, mechanistically layered therapies. Its pharmacology has opened a new conceptual doorway—one in which vascular biology, tissue regeneration, and reproductive endocrinology converge.
Conclusion
Sildenafil’s application in reproductive medicine epitomizes the intersection of theory and reality. Mechanistically, it makes perfect sense: by enhancing NO–cGMP signaling, it should improve uterine perfusion, thicken the endometrium, and facilitate implantation. Clinically, however, results remain modest, inconsistent, and context-dependent.
The drug’s paradox lies in its dependence on intact physiology. Sildenafil can amplify vascular and cellular signals, but it cannot create them ex nihilo. Its benefits likely manifest only in select patient subsets—those with functional, but underperforming, endometrial vasculature. In fibrotic or hormonally unresponsive tissues, its effects dissipate into pharmacologic silence.
Nonetheless, sildenafil’s legacy in gynecology is not failure but illumination. It has reframed how clinicians think about endometrial perfusion, vascular biology, and pharmacologic synergy. As reproductive medicine advances toward precision and personalization, sildenafil may yet find its place—not as a universal solution, but as a specialist tool in the broader orchestration of uterine receptivity.
FAQ: Sildenafil and Endometrial Receptivity
1. Why doesn’t sildenafil consistently improve pregnancy rates despite increasing blood flow?
Because endometrial receptivity depends on more than perfusion. Factors such as tissue integrity, hormonal balance, and molecular signaling often play a greater role than vascular flow alone.
2. Can sildenafil still benefit certain patients with thin endometrium?
Possibly yes. Women with demonstrable uterine hypoperfusion or endothelial dysfunction may respond to sildenafil, especially when used alongside estrogen or antioxidants. However, evidence remains limited and individualized assessment is key.
3. Is vaginal sildenafil safe for fertility patients?
Generally yes. Local administration minimizes systemic side effects and has not been associated with adverse reproductive outcomes. However, as efficacy is uncertain, it should be used within a monitored clinical protocol.