Introduction
Diabetes mellitus, once considered a disease of metabolism alone, has gradually revealed its pervasive grip on nearly every organ system. Among its less discussed but profoundly impactful complications lies male sexual dysfunction, a condition that affects up to 90% of men living with diabetes. Chronic hyperglycemia, vascular damage, neuropathy, and oxidative stress converge to sabotage male reproductive health—manifesting as erectile dysfunction, reduced libido, impaired spermatogenesis, and ultimately infertility.
While synthetic drugs like sildenafil citrate, a well-known phosphodiesterase type 5 (PDE5) inhibitor, have provided symptomatic relief, they do not address the metabolic and oxidative underpinnings of diabetic sexual dysfunction. Moreover, long-term use of synthetic PDE5 inhibitors raises concerns about cardiovascular safety, drug tolerance, and interaction with antihyperglycemic agents.
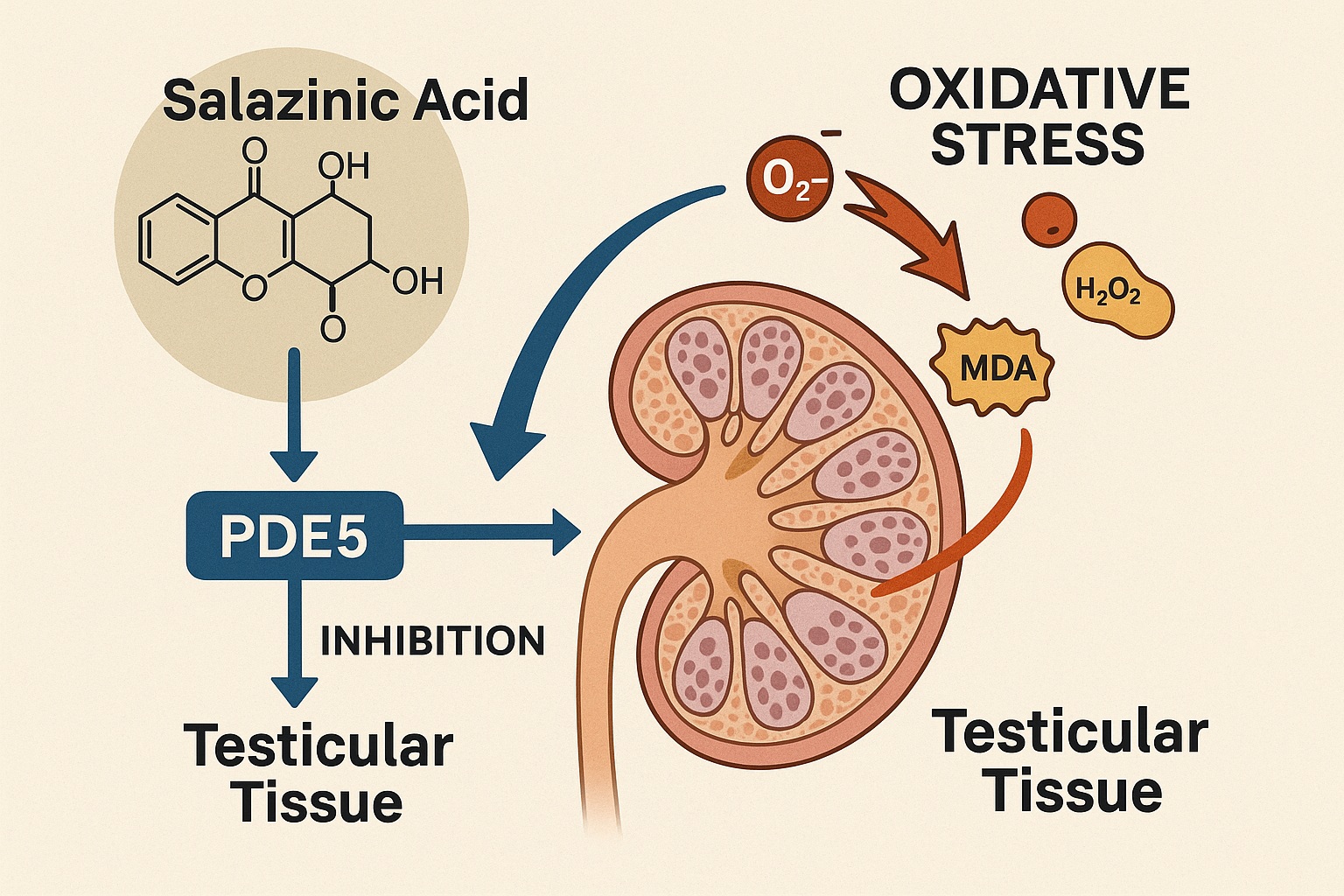
In this context, the scientific community has increasingly turned its gaze toward bioactive natural compounds capable of offering multifaceted protection—antioxidant, anti-inflammatory, and metabolic regulation—in addition to PDE5 inhibition. One such promising molecule is salazinic acid (Sa), a depsidone derivative abundantly present in lichen species such as Lobaria, Parmelia, and Usnea. The 2023 study by Killari et al. from Andhra University has illuminated the therapeutic potential of this compound, demonstrating its ability to restore sexual function, improve hormonal balance, and repair testicular tissue in diabetic rats.
This article delves into how salazinic acid not only rivals sildenafil in its molecular binding affinity for PDE5 but also extends beyond pharmacological mimicry to deliver broader metabolic benefits, making it a compelling candidate for future clinical translation.
The Burden of Diabetic Male Sexual Dysfunction
Male sexual dysfunction in diabetes arises from a complex interplay of vascular, hormonal, and oxidative mechanisms. Persistent hyperglycemia leads to glycation of endothelial proteins and subsequent microvascular dysfunction, impairing nitric oxide (NO) signaling—a key mediator of penile vasodilation. In parallel, reactive oxygen species (ROS) accumulation damages sperm DNA, disrupts Leydig cell steroidogenesis, and triggers apoptosis within the seminiferous tubules.
Erectile dysfunction, the most visible symptom, often coexists with less apparent impairments such as decreased sperm motility, abnormal morphology, and hormonal imbalances. The typical biochemical profile includes elevated malondialdehyde (MDA), reduced antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase), and diminished levels of testosterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH).
Despite the availability of PDE5 inhibitors that transiently enhance penile blood flow, they do little to reverse testicular oxidative injury or restore endocrine balance. Hence, the search for compounds that address the root biochemical derangements of diabetic sexual dysfunction—rather than simply alleviating symptoms—has intensified.
Salazinic Acid: Origin, Structure, and Biological Promise
Salazinic acid belongs to the depsidone family of lichen metabolites, characterized by their phenolic backbone and ability to form multiple hydrogen bonds with enzymatic proteins. Historically recognized for antioxidant, antimicrobial, and anti-inflammatory activities, salazinic acid has also shown α-glucosidase inhibitory properties, positioning it as a dual-action compound—capable of modulating both glucose metabolism and oxidative pathways.
Unlike synthetic drugs, salazinic acid operates in biochemical harmony with endogenous systems. It scavenges free radicals, reduces lipid peroxidation, and supports antioxidant enzyme regeneration. At the same time, its structural compatibility with PDE5’s catalytic domain allows it to inhibit cyclic guanosine monophosphate (cGMP) degradation, thereby facilitating smooth muscle relaxation and improving penile vascular dynamics.
This convergence of metabolic correction and vasodilatory action is what makes salazinic acid an intriguing natural counterpart to sildenafil—potentially offering sustained therapeutic benefits without the drawbacks of synthetic pharmacotherapy.
Experimental Insights: From Lichens to Laboratory Rats
In the study conducted by Killari and colleagues, male albino rats were rendered diabetic via streptozotocin (STZ) induction, a method that replicates type 1 diabetes by destroying pancreatic β-cells. These animals developed profound hyperglycemia, hormonal depletion, and testicular atrophy, mirroring human diabetic pathology.
Salazinic acid was administered orally at two concentrations—5 mg/kg and 10 mg/kg—over nine weeks, with sildenafil citrate (5 mg/kg) serving as a positive control. The results were nothing short of remarkable: salazinic acid restored body weight, reproductive organ mass, sperm quality, and hormonal balance in a dose-dependent manner.
In particular, the higher dose of salazinic acid matched or surpassed sildenafil in several endpoints:
- Fasting plasma glucose decreased by more than 50%.
- Serum insulin nearly doubled, indicating improved β-cell function.
- Testosterone, FSH, and LH levels normalized, supporting gonadal recovery.
- Antioxidant enzyme activity (SOD, GPx, CAT, and GSH) rose significantly, while MDA and TNF-α levels dropped, signifying reduced oxidative and inflammatory stress.
Perhaps most strikingly, sperm motility, viability, and count were restored to near-normal levels, and testicular histology revealed regenerated seminiferous tubules and Leydig cells.
These results affirm that salazinic acid acts not only as a PDE5 inhibitor but also as a metabolic modulator—restoring both the cause and consequence of diabetic male infertility.
Mechanistic Depth: How Salazinic Acid Works
Salazinic acid’s therapeutic effects stem from several interlinked molecular pathways.
1. PDE5 Inhibition and cGMP Preservation
Docking studies using AutoDock Vina revealed that salazinic acid binds strongly to the catalytic pocket of PDE5, with a binding affinity of −9.5 kcal/mol, slightly exceeding sildenafil’s (−9.2 kcal/mol). Both compounds share similar hydrogen bonding interactions—notably with Tyr612 and Gln817, residues crucial for stabilizing the PDE5–cGMP complex.
By competing with cGMP for the catalytic domain, salazinic acid effectively inhibits its degradation, leading to elevated cGMP levels and enhanced vasodilation. This biochemical action underlies its restorative effect on penile hemodynamics and sexual performance.
2. Antioxidant Defense and Oxidative Repair
Diabetic conditions generate a biochemical storm of superoxide radicals and lipid peroxides that impair sperm membrane integrity. Salazinic acid’s phenolic hydroxyl groups donate hydrogen atoms to neutralize free radicals, while also boosting endogenous antioxidant enzymes. This dual action not only curtails oxidative injury but also preserves mitochondrial integrity, ensuring sustained ATP production essential for sperm motility.
3. Anti-inflammatory Modulation
Chronic hyperglycemia activates TNF-α and other proinflammatory cytokines, which impair Leydig cell testosterone synthesis. Salazinic acid significantly suppressed TNF-α expression in diabetic rats, suggesting an upstream anti-inflammatory mechanism that complements its antioxidant capacity.
In essence, salazinic acid works as a multifunctional molecular repair agent—simultaneously targeting oxidative, inflammatory, and enzymatic derangements that underpin diabetic sexual dysfunction.
Comparative Performance: Salazinic Acid Versus Sildenafil
When benchmarked against sildenafil citrate, salazinic acid exhibited comparable or superior efficacy in multiple physiological parameters. While both compounds improved sexual behavior metrics such as mount frequency, intromission frequency, and ejaculation latency, salazinic acid provided additional systemic benefits that sildenafil could not.
Unlike sildenafil—which primarily acts locally on penile vasculature—salazinic acid demonstrated:
- Improved hepatic and renal biomarkers (ALT, AST, ALP, creatinine, urea).
- Normalized lipid profile, reducing total cholesterol and LDL while increasing HDL.
- No observed toxicity, even at tenfold doses in acute toxicity studies.
From a pharmacodynamic standpoint, this suggests salazinic acid’s action extends beyond PDE5 inhibition to encompass metabolic correction, hormonal reactivation, and organ protection—a combination rarely seen in synthetic analogs.
Testicular Recovery and Histological Regeneration
Microscopic evaluation of testicular tissue revealed profound architectural recovery in salazinic acid–treated rats. Diabetic controls displayed disorganized seminiferous tubules, widened interstitial spaces, and degeneration of spermatogenic cells, with an almost complete absence of Leydig cells.
Conversely, treatment with salazinic acid—particularly at 10 mg/kg—restored germinal epithelium integrity, reduced interstitial gaps, and reestablished spermatogenic order. Leydig cell populations reappeared, correlating with restored testosterone synthesis. The Mean Johnson’s score (a metric of spermatogenic health) rose from 4.3 in diabetic rats to 9.1 in the high-dose salazinic acid group—nearly equivalent to healthy controls.
This regeneration is a compelling indicator that salazinic acid’s benefits are not merely biochemical but also histological, reinforcing its candidacy as a natural testicular protectant.
In Silico and In Vitro Convergence: Molecular Validation
Computational modeling strengthens the case for salazinic acid’s pharmacological relevance. Molecular dynamics simulations demonstrated that the salazinic acid–PDE5 complex remained stable over 40 nanoseconds, with consistent hydrogen bonding and minimal root-mean-square deviation (RMSD < 3 Å).
Further, MMGBSA free-energy calculations confirmed stable binding, with ΔG values comparable to sildenafil. In vitro PDE5 inhibition assays yielded an IC₅₀ of 8.16 nM for salazinic acid, closely aligning with sildenafil’s 5.77 nM. These parallel findings across computational and experimental domains confirm that salazinic acid is not a weak mimic but a potent, structurally valid PDE5 inhibitor.
Equally important, ADMET predictions confirmed excellent drug-likeness, minimal toxicity, and good gastrointestinal absorption. The compound showed no Pan-Assay Interference (PAINS) alerts and negligible hepatotoxic or carcinogenic potential—a major advantage over synthetic analogs.
Translational Outlook: Toward Human Therapeutics
The implications of these findings extend well beyond the laboratory. Salazinic acid’s dual-action profile—antidiabetic and pro-reproductive—positions it as a unique pharmacological bridge between metabolic and sexual health. In an era where diabetes prevalence continues to soar, affecting over 420 million people globally, the need for multitarget natural therapeutics is both urgent and unmet.
In potential human application, salazinic acid could serve:
- As a natural adjunct to PDE5 inhibitors, enhancing efficacy and reducing required dosage.
- As a standalone phytopharmaceutical for mild-to-moderate diabetic erectile dysfunction.
- As a protective supplement against testicular oxidative damage in men with chronic metabolic disorders.
Future translational work should focus on bioavailability optimization, pharmacokinetic profiling, and controlled clinical trials to establish safety and dosing in humans. Given its nontoxic profile and multi-system benefits, salazinic acid may represent a paradigm shift in the management of diabetic male infertility, moving therapy from symptomatic relief to true restoration of physiological balance.
Conclusion
The investigation into salazinic acid’s pharmacological potential reveals more than a lichen-derived curiosity—it introduces a credible candidate for natural, multi-mechanistic intervention in diabetic male sexual dysfunction.
By merging antioxidant, anti-inflammatory, and PDE5-inhibitory mechanisms, salazinic acid demonstrates a rare versatility: it normalizes glucose, protects organs, restores hormonal function, and revives fertility markers. Its equivalence to sildenafil in enzymatic inhibition—coupled with superior systemic safety—makes it a compelling focus for further research.
As biomedical science continues to seek safer, smarter, and more integrative solutions to metabolic disease, salazinic acid stands as a reminder that nature’s chemical ingenuity often outpaces our own. The humble lichen, it seems, may hold the key to revitalizing both metabolic and reproductive health—an elegant intersection of ecology and endocrinology.
FAQ: Salazinic Acid and Diabetic Male Infertility
1. How does salazinic acid differ from sildenafil in treating erectile dysfunction?
While sildenafil acts primarily by inhibiting PDE5 in penile tissue, salazinic acid also improves metabolic health, reduces oxidative stress, and restores hormonal balance. It provides systemic recovery rather than short-term vascular relaxation.
2. Is salazinic acid safe for human use?
Preclinical toxicity data indicate that salazinic acid is well tolerated up to 100 mg/kg, with no observable organ damage or behavioral changes. In silico predictions suggest low toxicity and good drug-likeness, but clinical trials are needed to confirm safety in humans.
3. Could salazinic acid be developed into a prescription drug or supplement?
Potentially yes. Its strong PDE5 binding, metabolic benefits, and natural origin make it a promising phytopharmaceutical candidate. Future research should focus on formulation, bioavailability, and human pharmacokinetics to enable clinical translation.