Introduction
Few drugs have journeyed from the realm of cardiovascular medicine to neonatal and fetal therapy with such intrigue as sildenafil citrate. Originally heralded for its vasodilatory prowess in pulmonary hypertension and its more infamous association with erectile dysfunction, sildenafil has reemerged as a potential prenatal therapeutic for several fetal disorders, including congenital diaphragmatic hernia (CDH), intrauterine growth restriction (IUGR), and preeclampsia.
However, enthusiasm for antenatal sildenafil was tempered after the early termination of the STRIDER trial, in which fetuses exposed to the drug displayed increased perinatal mortality. The controversy underscored a pressing need for rigorous preclinical evaluation of fetal pharmacokinetics and pharmacodynamics—not in rodents or cell cultures, but in large, physiologically relevant animal models that reflect human development.
It is within this context that De Bie et al. (2021) introduced a novel experimental paradigm: the EXTra-uterine Environment for Neonatal Development (EXTEND), a system that mimics the womb yet offers direct access to the fetus. This “artificial placenta” model enables controlled intravenous drug delivery, bypassing the complexities of maternal metabolism and placental transfer. Using fetal lambs, whose cardiovascular and pulmonary maturation parallels that of human fetuses, the researchers probed how sildenafil behaves when administered directly to the developing organism.
Their findings provide a rare window into fetal pharmacology, illuminating not only the dose–response relationships and safety margins of sildenafil but also the methodological innovations required to study such effects in utero.
Rationale for Fetal Sildenafil Therapy
Sildenafil’s appeal in fetal medicine lies in its dual mechanism:
- Vasodilation, via inhibition of phosphodiesterase type 5 (PDE5), prevents degradation of cyclic guanosine monophosphate (cGMP), enhancing nitric oxide–mediated smooth muscle relaxation.
- Anti-remodeling, by attenuating the proliferation of vascular smooth muscle cells, can modify pathological pulmonary vascular development.
These effects are particularly valuable in conditions characterized by fetal pulmonary hypertension—notably CDH, where hypoplastic lungs and maladaptive vascular growth threaten postnatal survival. Preclinical studies in rodents and rabbits have shown that antenatal sildenafil mitigates pulmonary arterial thickening and improves postnatal oxygenation. Yet the translation of these results into humans has proven problematic.
Sheep, with their large fetuses and human-like cardiovascular physiology, represent the logical bridge between laboratory and clinic. But an unexpected obstacle emerged: transplacental transfer of sildenafil in sheep is minimal, with maternal dosing yielding fetal plasma levels far below therapeutic thresholds. Hence, direct fetal administration became necessary—a task only feasible through extracorporeal circulation models such as EXTEND.
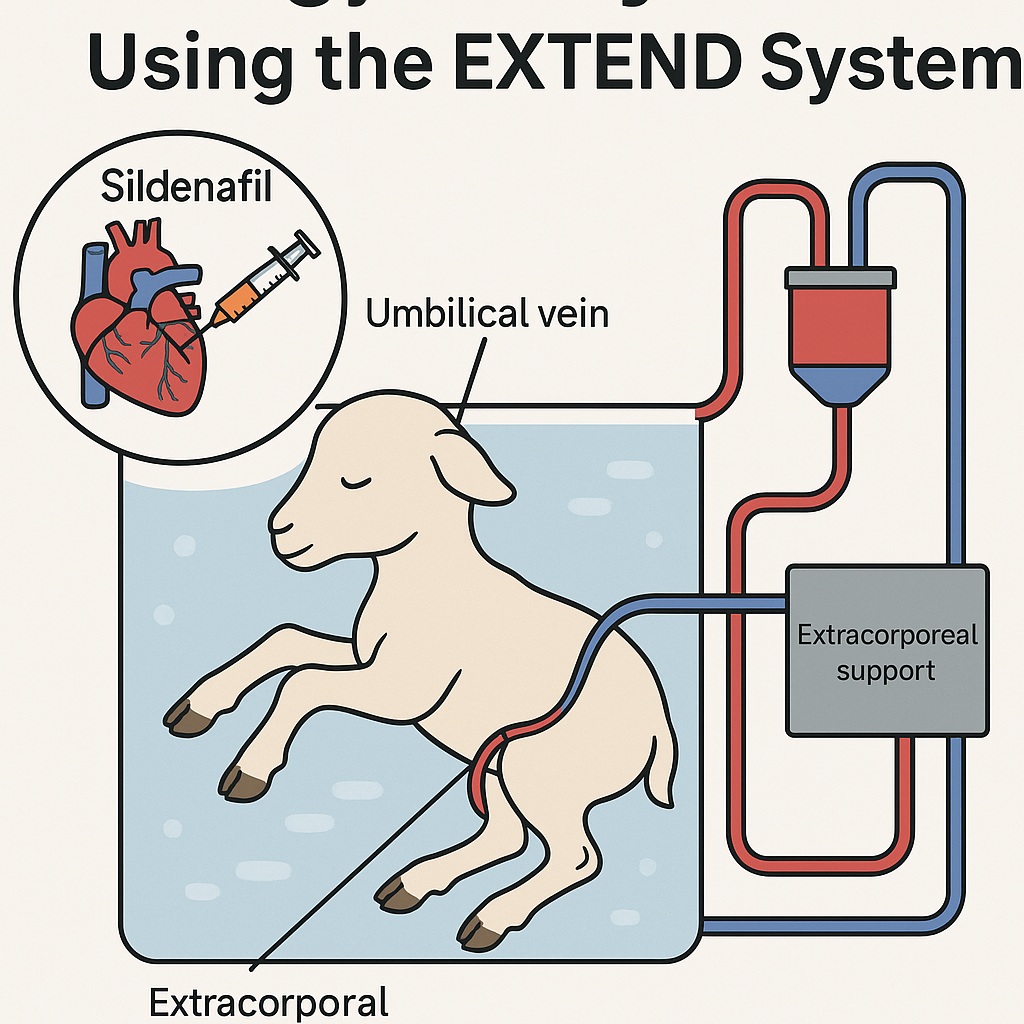
The EXTEND Model: A Controlled Womb Outside the Womb
The EXTEND system is an elegant fusion of neonatal physiology and bioengineering. In this setup, a premature fetal lamb is delivered via cesarean section and connected to a pumpless extracorporeal oxygenator through the umbilical vessels. The animal remains suspended in a sterile, temperature-controlled, amniotic-like fluid bath. Gas exchange occurs via the oxygenator rather than the maternal placenta, while parenteral nutrition sustains growth.
This setup maintains fetal circulation patterns—including a patent ductus arteriosus and foramen ovale—while allowing real-time hemodynamic monitoring. For researchers, it provides a rare opportunity to observe, manipulate, and quantify fetal physiological responses over days or weeks without maternal interference.
By infusing sildenafil directly into the umbilical vein, the EXTEND model eliminates confounding factors such as maternal metabolism, placental degradation, and variable transplacental permeability. This isolation enables an unambiguous assessment of sildenafil’s pharmacokinetics (PK)—how the fetal body processes the drug—and pharmacodynamics (PD)—how the fetal circulation reacts to it.
Pharmacokinetics of Sildenafil in Fetal Lambs
The study examined three continuous infusion regimens—0.3, 0.5, and 0.7 mg/kg/24 hours—administered intravenously through the umbilical vein. Blood samples collected at precise intervals yielded a robust PK profile, later modeled using population pharmacokinetic techniques.
Steady-State Behavior and Drug Clearance
Sildenafil’s steady-state concentration was achieved within the clinical target range (47–500 ng/mL) for all doses tested. However, the half-life in fetal lambs proved astonishingly long—43 hours, compared to 3–5 hours in adult humans. This finding implies markedly reduced metabolic clearance in the fetus, likely due to immature hepatic enzyme systems.
The absence of detectable desmethyl-sildenafil, a major active metabolite in adult humans, further supports the notion that fetal metabolic pathways differ fundamentally from postnatal ones. Such divergence complicates the extrapolation of adult dosing regimens to fetuses and underscores the necessity of species- and stage-specific pharmacokinetic studies.
Minimal Circuit Adsorption
Given sildenafil’s lipophilic nature (Log P = 2.75), there was initial concern that the drug might adhere to the tubing and oxygenator of the extracorporeal circuit—a phenomenon known to distort PK analyses in extracorporeal membrane oxygenation (ECMO). Yet ex vivo tests demonstrated negligible adsorption, confirming that EXTEND’s bioactive polymer-coated surfaces maintain chemical neutrality.
This technical reassurance validated subsequent in vivo measurements, ensuring that observed concentration dynamics truly reflected fetal disposition rather than system artifact.
Pharmacodynamic Insights: Sildenafil’s Hemodynamic Signature
Understanding how sildenafil alters fetal cardiovascular behavior was the core aim of the study. Using continuous hemodynamic monitoring and high-resolution Doppler ultrasonography, researchers evaluated pulmonary vascular resistance (PVR), arterial pressures, and circuit flow dynamics.
Pulmonary Effects
Across most administrations, sildenafil induced a prompt reduction in pulmonary vascular resistance, evidenced by an increased acceleration time to ejection time ratio (AT/ET) in the right pulmonary artery. The effect manifested within two hours of infusion and subsided after 24–48 hours. Importantly, this vasodilation occurred at plasma concentrations well below those typically required in adults—a testament to the fetus’s heightened vascular responsiveness.
Furthermore, the ratio of ductus arteriosus output to right ventricular stroke volume (DAO/RVSV) declined, suggesting blood flow was preferentially redistributed toward the pulmonary circulation. These findings mirror sildenafil’s intended therapeutic goal in CDH: enhancing pulmonary perfusion and promoting vascular remodeling.
However, an intriguing outlier emerged—one animal exhibited paradoxical pulmonary vasoconstriction. Whether this reflects interindividual variation, gestational stage differences, or a yet-unknown compensatory mechanism remains uncertain, but it serves as a cautionary reminder of fetal heterogeneity.
Systemic Effects
Sildenafil’s systemic actions were transient but measurable. Mean arterial pressure and circuit flow both decreased shortly after infusion, with effects lasting longer at higher doses. Despite these shifts, biochemical parameters—lactate and pH—remained stable, indicating that tissue perfusion and oxygenation were not compromised.
This hemodynamic pattern—a self-limiting hypotension without metabolic acidosis—suggests good acute fetal tolerance. The 0.5 mg/kg/24 h dose emerged as the optimal balance, achieving target concentrations rapidly while minimizing systemic perturbations.
Physiological Interpretation: Why the Fetal Response Differs
The fetal circulation is a unique physiological system, designed to shunt blood away from the lungs toward the placenta. Introducing a potent pulmonary vasodilator like sildenafil challenges this equilibrium. In the EXTEND model, where gas exchange occurs extracorporeally rather than via the placenta, the fetus’s pulmonary circulation suddenly becomes more functionally relevant.
The observed transient vasodilation likely reflects an adaptive reflex—an attempt by the fetal cardiovascular system to restore its familiar pressure gradients. In short, the fetus briefly “tests” pulmonary blood flow before reasserting its baseline distribution. Such autoregulatory behavior may explain why even increasing sildenafil concentrations did not prolong vasodilation indefinitely.
The long fetal half-life of sildenafil also means that repeated or continuous exposure could lead to accumulation, potentially exaggerating systemic hypotension if dosing is not carefully calibrated. These considerations are critical for any future clinical translation of fetal pharmacotherapy, where narrow safety margins are the rule, not the exception.
Safety Considerations and Ethical Dimensions
Animal welfare was meticulously safeguarded under institutional and federal guidelines, and the EXTEND model itself offers an ethical refinement by reducing maternal morbidity and fetal mortality compared with traditional intrauterine experiments. Still, extrapolating these results to human pregnancy demands caution.
The developing fetus is not merely a small adult; it is a dynamic organism undergoing rapid organogenesis. Pharmacological interventions at this stage can yield lifelong consequences—beneficial or otherwise. While sildenafil appears biochemically well-tolerated in the short term, long-term data on organ development, neurocognitive outcomes, and retinal effects remain sparse.
The authors themselves acknowledged these gaps and highlighted the need for extended studies assessing hepatic, neurological, and pulmonary tissue integrity following chronic exposure.
Methodological Strengths: Precision in a Miniature Physiology
From a research methodology standpoint, this work exemplifies meticulous experimental design. Several key strengths stand out:
- Direct fetal access through the EXTEND system eliminates confounding maternal metabolism.
- Continuous monitoring of pressures, flows, and oxygen saturation enables high temporal resolution of hemodynamic changes.
- Population PK modeling allows extrapolation from limited animal numbers while preserving statistical robustness.
Moreover, by using historical control data from unmedicated lambs, the investigators established reference variability ranges, enabling clear discrimination between drug-induced effects and spontaneous fluctuations.
These methodological advances may redefine how future investigators approach prenatal pharmacology, paving the way for systematic dose-finding and safety studies of other candidate drugs.
Translational Implications: From Lambs to Human Fetuses
The ultimate goal of such research is not merely to understand sheep physiology but to refine fetal therapy in humans. The EXTEND model’s success in sustaining fetal life outside the womb for weeks holds extraordinary translational potential. It can serve as a testing platform for drugs, devices, and interventions before exposing human fetuses to unknown risks.
For sildenafil specifically, these results provide an empirical basis for recalibrating dosing strategies. The 0.5 mg/kg/24 h regimen offers a reference point for achieving therapeutic plasma levels while maintaining hemodynamic stability. Moreover, the finding that effective pulmonary vasodilation occurs at concentrations below adult IC₅₀ levels suggests that lower fetal doses may suffice, mitigating systemic side effects.
Nevertheless, human translation requires confronting several uncertainties:
- Species-specific differences in hepatic enzyme expression and placental physiology.
- Developmental variations in PDE5 receptor density and vascular sensitivity.
- Long-term developmental effects of prenatal vasodilator exposure.
The EXTEND approach cannot perfectly replicate the maternal–fetal interface, but it brings the field one significant step closer to precision prenatal pharmacotherapy—where interventions are guided by direct evidence rather than extrapolation and hope.
Limitations and Future Directions
Every pioneering study opens as many questions as it answers. De Bie et al. candidly acknowledged several limitations:
- Small sample size (n = 5) due to pandemic-related constraints limited statistical generalizability, though modeling mitigated this issue.
- Non-invasive monitoring, while safer, cannot match the granularity of invasive cardiac catheterization for pressure measurements.
- Lack of central oxygen tension data hindered precise correlation between oxygenation and PVR changes.
- Short study duration precluded evaluation of chronic or developmental effects.
Future investigations should therefore integrate longitudinal tissue analyses, include pathological fetal models (such as CDH), and perhaps even explore combination therapies that pair sildenafil with agents influencing angiogenesis or oxidative stress.
The study also raises philosophical questions about the future of the EXTEND model itself. Could this technology one day serve as an intermediate incubator for extremely preterm infants, offering a bridge between womb and NICU? If so, pharmacological data obtained in fetal lambs may soon inform dosing protocols for premature humans, reshaping the boundaries of perinatal medicine.
Conclusion
The exploration of sildenafil in fetal lambs on extracorporeal support is more than an isolated pharmacological experiment—it is a blueprint for the future of fetal therapy. By merging rigorous pharmacokinetics with physiological insight and engineering innovation, the study demonstrates that safe and measurable drug delivery to the fetus is both possible and informative.
The key takeaways are clear:
- Sildenafil induces transient pulmonary vasodilation and mild systemic hypotension without biochemical harm.
- The 0.5 mg/kg/24 h dose optimally balances efficacy and safety.
- The EXTEND system offers an unprecedented platform for controlled fetal drug research, free from maternal and placental interference.
While caution remains essential, the findings invite cautious optimism. With further refinement, such models could usher in a new era of rational, evidence-based fetal pharmacotherapy, transforming how we approach congenital disease before birth.
In a sense, sildenafil’s journey from adult vasodilator to fetal therapeutic mirrors modern medicine’s trajectory—from treating disease after birth to preventing it before life even begins.
FAQ: Key Questions About Fetal Sildenafil Research
1. Why study sildenafil in fetal lambs instead of directly in humans?
Because fetal physiology cannot ethically or safely be studied in utero, fetal lambs serve as a close analogue. Their cardiovascular and pulmonary systems closely resemble those of human fetuses, allowing safe exploration of dosing, safety, and pharmacodynamics before clinical trials.
2. What makes the EXTEND system so revolutionary?
EXTEND recreates a womb-like environment ex utero, allowing continuous fetal development while enabling direct access to the circulation. It bypasses maternal metabolism, offering unprecedented control and observation of fetal responses to drugs and interventions.
3. Does this research mean sildenafil will be used again in human pregnancies?
Not immediately. The study’s purpose was to clarify mechanisms and safety parameters, not to endorse clinical use. However, by establishing pharmacological foundations and identifying safe dosing windows, it provides critical groundwork for future, tightly regulated human research.