Introduction
Erectile dysfunction (ED) remains one of the most prevalent male health concerns worldwide, affecting an estimated 300 million men by 2025. It is no longer viewed merely as a sexual limitation but as a complex, multifactorial condition intertwined with vascular, endocrine, and psychological dysfunctions. Conventional therapeutics — most notably phosphodiesterase-5 (PDE-5) inhibitors like sildenafil — have revolutionized treatment, but they come at the cost of adverse effects such as headache, flushing, hypotension, dyspepsia, and even visual disturbances. For many patients, these side effects hinder adherence and satisfaction, driving a growing demand for natural alternatives with fewer complications and greater holistic benefits.
Enter Spondias mangifera, a modest fruit-bearing tree belonging to the Anacardiaceae family, known in traditional Indian medicine as Amrata. Historically used in Ayurveda and Unani medicine, nearly every part of this tree — leaves, bark, roots, and fruit — has been exploited for its curative properties. The fruit, in particular, has long been used as a nutraceutical rich in vitamins, organic acids, and bioactive phytochemicals. Recently, pharmacological research has begun to validate these traditional claims, revealing that S. mangifera may harbor potent aphrodisiac and PDE-5 inhibitory properties.
This article delves into the biochemical, molecular, and behavioral evidence supporting S. mangifera’s role as a natural PDE-5 inhibitor and sexual function enhancer. Drawing from an integrated in silico, in vitro, and in vivo approach, we explore how its bioactive compounds — particularly β-amyrin, β-sitosterol, and oleanolic acid — emulate the effects of sildenafil in rodents, potentially offering a safer, plant-based alternative for male sexual dysfunction.
The Pharmacological Rationale for Natural Aphrodisiacs
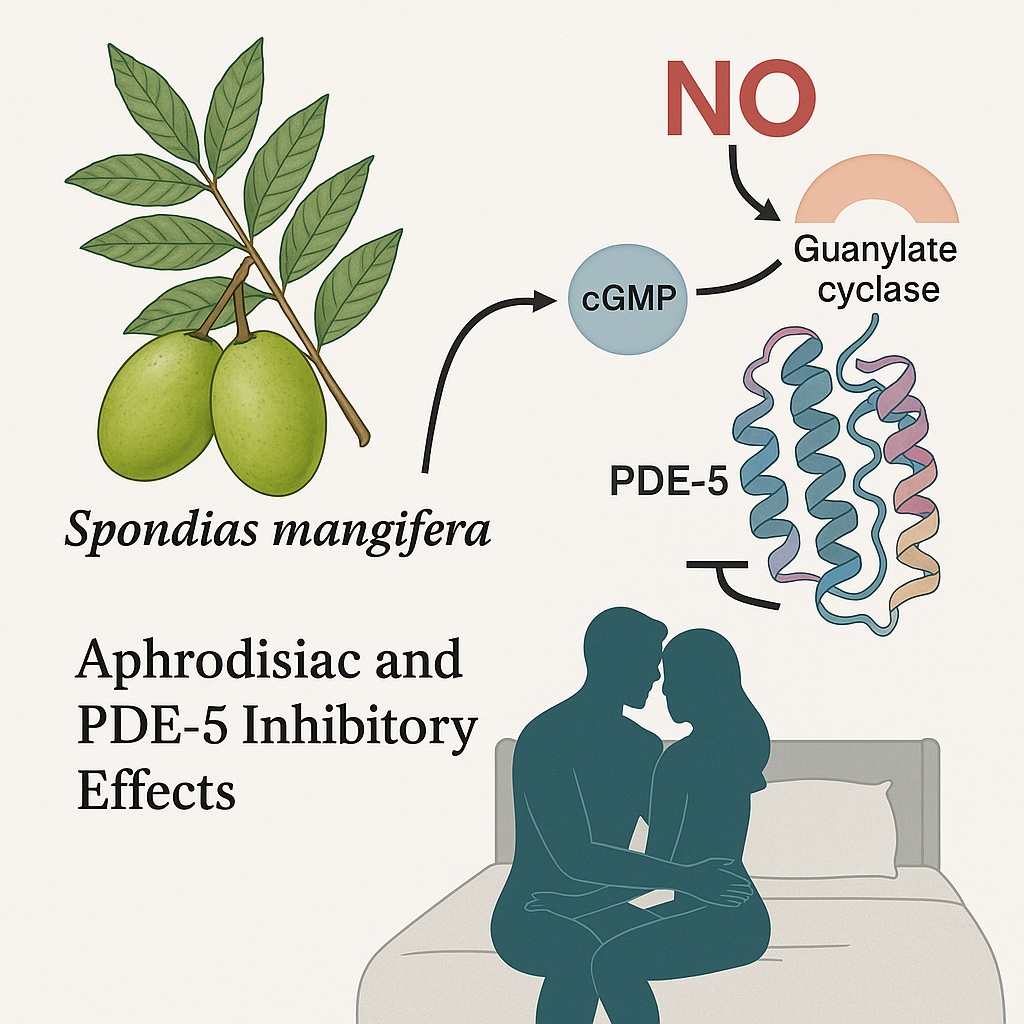
Human sexual behavior is governed by a sophisticated network involving the hypothalamic–pituitary–gonadal axis, the autonomic nervous system, and local vascular dynamics. Any disruption in this delicate interplay — through aging, metabolic disorders, or psychosocial stress — can lead to ED. The mechanism of penile erection fundamentally depends on the nitric oxide (NO)–cyclic guanosine monophosphate (cGMP) pathway. Here, NO released from endothelial cells activates guanylate cyclase, which increases cGMP levels, leading to smooth muscle relaxation and increased penile blood flow.
However, the enzyme phosphodiesterase-5 (PDE-5) rapidly degrades cGMP, terminating the erection process. Sildenafil, a selective PDE-5 inhibitor, prevents this breakdown and prolongs the erectile response. Yet, it acts systemically, often causing non-selective vasodilation and undesired cardiovascular effects. Therefore, natural PDE-5 inhibitors with selective activity and minimal toxicity represent an appealing frontier.
Traditional herbal medicine offers a vast repertoire of potential PDE-5 modulators. Extracts of Ginkgo biloba, Vitis vinifera, and Polygonatum verticillatum have shown inhibitory activity, while plants like Allium tuberosum and Carpolobia alba improve sexual behavior in animal models. Spondias mangifera joins this lineage — supported not by anecdote alone but by rigorous pharmacological and computational data.
Bioactive Composition of Spondias mangifera
Phytochemical studies have identified several active constituents in S. mangifera fruit and aerial parts:
- β-amyrin – a pentacyclic triterpene with known anti-inflammatory, antioxidant, and neuroactive properties.
- β-sitosterol – a phytosterol involved in hormonal modulation and cholesterol metabolism.
- Oleanolic acid – a triterpenoid compound with hepatoprotective and vasodilatory effects.
These compounds collectively exhibit lipophilic affinity for cellular membranes and possess hydrophobic surfaces suitable for enzyme binding. In traditional Indian systems, fruit pulp and juice have been used to boost vitality, treat dysentery, diabetes, and rheumatism, and alleviate symptoms related to reproductive fatigue — observations now supported by modern mechanistic evidence.
Molecular Docking and In Silico Insights
The pharmacological hypothesis that S. mangifera’s aphrodisiac activity stems from PDE-5 inhibition was tested through molecular docking and dynamic simulation studies using AutoDock Vina and GROMACS platforms. Crystal structures of PDE-5 (PDB ID: 2H42) and dopamine receptor D2 (PDB ID: 6CM4) were employed to model ligand binding.
Among the identified bioactives:
- β-amyrin exhibited the strongest binding affinity (−11.61 kcal/mol), surpassing even sildenafil (−10.01 kcal/mol).
- Oleanolic acid and β-sitosterol also showed substantial affinity (−10.51 and −9.90 kcal/mol, respectively).
These compounds formed hydrogen bonds with key catalytic residues (SER663, ASN662, ILE665) and hydrophobic interactions with residues (LEU765, PHE820, TYR612) critical for PDE-5 inhibition. Their binding orientation mimicked sildenafil’s active conformation, validating their structural complementarity to the PDE-5 pocket.
Molecular dynamics simulations further confirmed complex stability over 100 nanoseconds, with β-amyrin–PDE-5 complexes demonstrating lower root mean square deviation (RMSD) and potential energy than sildenafil complexes. These results suggest that β-amyrin may sustain a more stable and energetically favorable interaction, reinforcing its status as the lead natural inhibitor.
In Vitro and In Vivo Evaluation: From Molecule to Behavior
To translate computational promise into biological relevance, researchers conducted a series of in vitro enzyme assays and in vivo behavioral experiments in male rodents. Ethanolic extracts of S. mangifera fruit (SMFE) were administered orally at 100, 200, and 400 mg/kg body weight for 28 days, with sildenafil (5 mg/kg) serving as a reference standard.
Behavioral Parameters
Male sexual performance was quantified using established metrics:
- Mount frequency (MF) – number of mounts before ejaculation.
- Intromission frequency (IF) – number of vaginal penetrations.
- Ejaculation frequency (EF) and latency (EL) – indicators of stamina and satisfaction.
- Mount latency (ML) and intromission latency (IL) – time to first sexual act, reflecting arousal speed.
At doses of 200 and 400 mg/kg, SMFE significantly increased MF, IF, and EF (p < 0.05), while reducing ML and IL — indicating heightened libido and improved performance. Ejaculation latency was prolonged, mirroring the ejaculatory delay seen with sildenafil, suggesting enhanced endurance.
Interestingly, the 100 mg/kg dose did not yield significant changes, suggesting a threshold effect for pharmacological efficacy.
Biochemical Correlates
Sexual performance improvements were paralleled by biochemical shifts:
- PDE-5 activity in penile tissue decreased significantly (p < 0.05).
- Nitric oxide levels and testosterone concentrations increased markedly, establishing a link between molecular inhibition and physiological outcome.
These findings demonstrate that SMFE acts not merely as a central stimulant but as a true peripheral enhancer of erectile function through NO–cGMP signaling.
Mechanistic Integration: The Triad of Action
The therapeutic activity of S. mangifera fruit extract can be attributed to a tripartite mechanism:
- 1. PDE-5 inhibition: β-amyrin and oleanolic acid competitively inhibit PDE-5, elevating cGMP levels and sustaining vasodilation in corpus cavernosum tissue.
- 2. Nitric oxide upregulation: The extract enhances endothelial NO synthesis, improving vascular tone and responsiveness.
- 3. Hormonal modulation: Elevated serum testosterone augments sexual desire and facilitates the neural reflexes governing erection.
Together, these mechanisms echo the pharmacodynamics of sildenafil, albeit via naturally balanced modulation rather than abrupt enzymatic blockade. The result is improved sexual performance without systemic strain — a hallmark advantage of phytotherapeutics.
Comparative Perspective: S. mangifera vs. Sildenafil
While sildenafil’s clinical efficacy is undisputed, its pharmacological precision often translates into systemic side effects. S. mangifera, in contrast, presents a more gradual yet holistic effect.
Unlike synthetic PDE-5 inhibitors, SMFE exerts multi-level modulation — simultaneously supporting NO production, hormone balance, and antioxidant defense.
Key distinctions include:
- Safety: No adverse effects on cardiovascular parameters or behavior were noted in animal trials.
- Sustainability: Derived from edible fruits, SMFE may serve as a dietary supplement, not merely a pharmacological agent.
- Adaptogenic potential: The fruit’s antioxidants (vitamin C, β-sitosterol) may protect reproductive tissues from oxidative stress, a major factor in age-related ED.
While S. mangifera cannot yet replace sildenafil in acute ED management, it may complement pharmacotherapy or serve as a preventive nutraceutical promoting long-term sexual wellness.
The Molecular Poetry of Plant-Based Drug Design
From a medicinal chemistry standpoint, S. mangifera exemplifies the elegance of nature-inspired pharmacology. Its triterpenes and phytosterols mirror the hydrophobic scaffolds that medicinal chemists painstakingly design in laboratories. β-amyrin’s rigid pentacyclic backbone provides a stable binding surface, while oleanolic acid’s polar groups facilitate hydrogen bonding and spatial complementarity within PDE-5’s catalytic domain.
Modern computational pharmacognosy leverages such natural architectures, employing docking and dynamic simulation to predict efficacy before synthesis. S. mangifera demonstrates how ethnomedicine can inform rational drug discovery, transforming ancient remedies into modern, evidence-based solutions.
The Promise of Nutraceutical Aphrodisiacs
The current pharmacological landscape increasingly favors nutraceuticals — substances that bridge nutrition and therapy. S. mangifera fruit extract represents a paradigm shift toward functional aphrodisiacs, offering not only enhanced sexual performance but also systemic metabolic benefits.
In diabetic models, for instance, the extract’s α-glucosidase inhibition supports glucose homeostasis, mitigating hyperglycemia-induced erectile dysfunction. Its antioxidant capacity shields sperm DNA from oxidative insult, thereby improving fertility indices. Thus, the extract’s benefits extend beyond erection, addressing broader reproductive health.
The convergence of metabolic regulation, hormonal balance, and vascular enhancement positions S. mangifera as a multi-targeted sexual health agent, reflecting the holistic vision of traditional medicine in a pharmacologically validated framework.
Limitations and Future Directions
Despite encouraging results, several gaps remain before S. mangifera can transition from preclinical promise to clinical application. Key considerations include:
- Pharmacokinetic profiling: The bioavailability and tissue distribution of β-amyrin, β-sitosterol, and oleanolic acid require detailed characterization.
- Dose standardization: Variability in extract preparation may influence potency; standardized formulations must be developed.
- Clinical trials: Rigorous, placebo-controlled human studies are essential to confirm safety and efficacy in ED management.
Emerging research might also explore synergistic formulations, combining S. mangifera extract with other natural PDE-5 inhibitors such as Ginkgo biloba or Tribulus terrestris. Moreover, encapsulation within nanocarriers or phospholipid complexes could enhance bioavailability, enabling targeted penile delivery and sustained pharmacological action.
Conclusion
The rediscovery of Spondias mangifera as a potential natural aphrodisiac underscores the untapped pharmacological wealth hidden within traditional botanicals. From molecular docking to behavioral validation, every layer of evidence points toward its ability to inhibit PDE-5, boost nitric oxide, and elevate testosterone — mechanisms mirroring those of established ED therapeutics but achieved through natural harmony.
In an age seeking balance between efficacy and safety, S. mangifera fruit extract offers a compelling alternative: a biocompatible, multi-mechanistic, and culturally rooted solution for sexual health restoration. It stands not merely as a substitute for synthetic drugs but as a symbol of pharmacological integration — where tradition meets precision science, and nature meets necessity.
FAQ: Key Questions About Spondias mangifera and Its Aphrodisiac Potential
1. Can Spondias mangifera fruit extract really improve sexual performance?
Yes. In controlled animal studies, ethanolic fruit extract enhanced mount, intromission, and ejaculation frequencies while reducing latency periods. These behavioral improvements correlated with increased nitric oxide and testosterone levels and decreased PDE-5 activity — mechanisms identical to those of sildenafil.
2. Is S. mangifera safe compared to conventional PDE-5 inhibitors?
Preclinical results indicate no observable toxicity or adverse cardiovascular effects, even at higher doses (400 mg/kg). However, human safety data are still limited, and clinical trials are required before broad therapeutic use.
3. How does S. mangifera differ from other herbal aphrodisiacs?
Unlike herbs that act primarily as hormonal stimulants, S. mangifera targets the biochemical root of erectile physiology — PDE-5 inhibition and cGMP enhancement — while simultaneously offering antioxidant and metabolic support. This makes it a unique, multifaceted candidate for both treatment and prevention of erectile dysfunction.