The treatment of erectile dysfunction (ED) has long relied on oral phosphodiesterase type 5 (PDE5) inhibitors—most notably, sildenafil citrate. Since its debut in the late 1990s, sildenafil has revolutionized sexual medicine, transforming an often stigmatized disorder into a treatable physiological condition. Yet, despite its undeniable clinical value, oral sildenafil is far from perfect. Its delayed onset, gastrointestinal degradation, and hepatic first-pass metabolism contribute to variable efficacy and dose-dependent side effects such as headache, flushing, and dyspepsia.
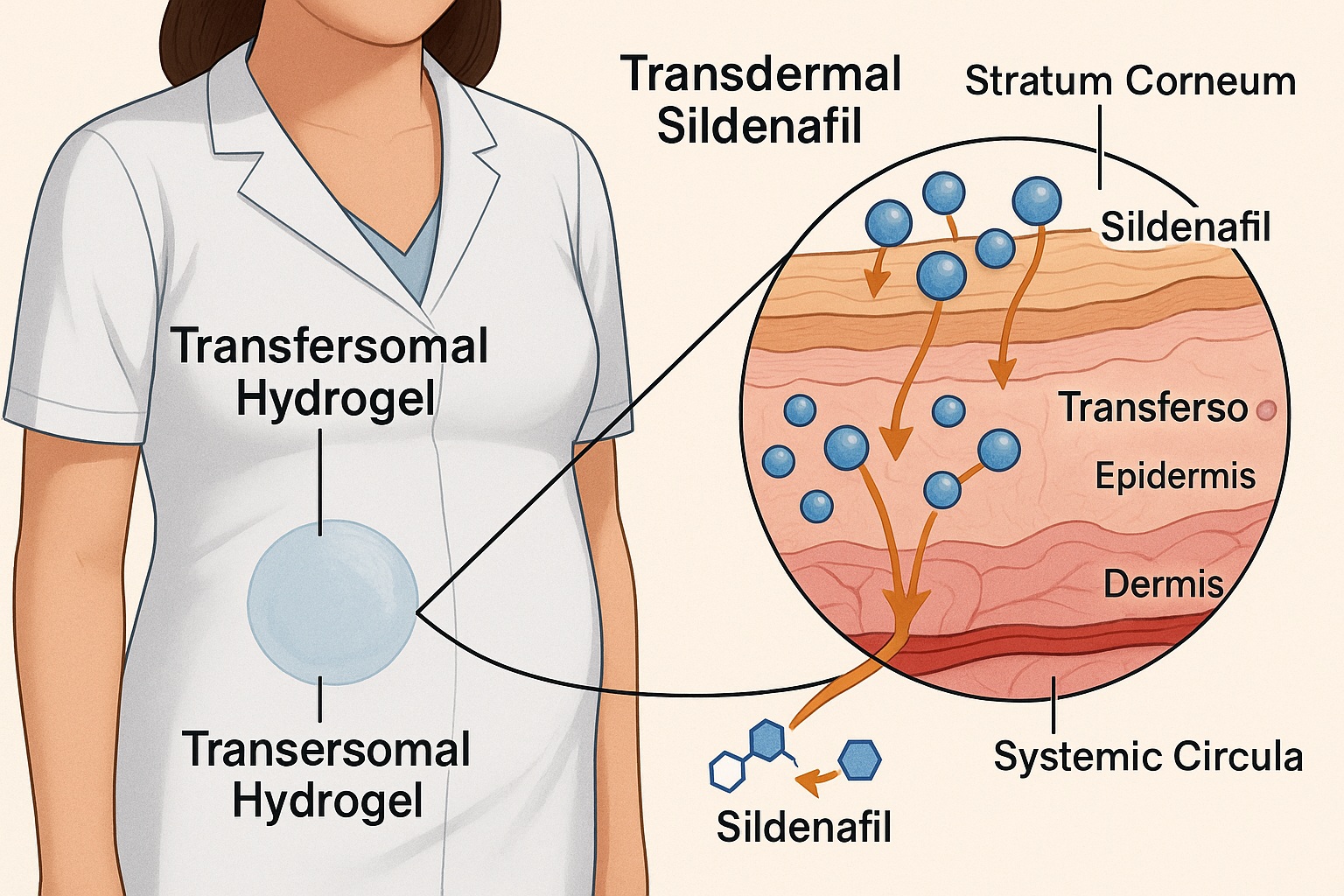
The pharmaceutical sciences have been quietly tackling these problems, and one of the most promising innovations to emerge is the transdermal delivery of sildenafil using transfersomal hydrogel technology. In essence, it’s the same molecule—but delivered smarter, faster, and safer. By bypassing the digestive tract and liver, this novel formulation offers higher bioavailability, prolonged systemic effect, and improved tolerability, all while maintaining therapeutic efficacy.
This is not merely a reformulation—it’s a rethinking of how ED therapy can fit the realities of patient behavior, comorbidities, and preferences. For clinicians, understanding this evolving technology is essential to anticipating how sildenafil may soon be administered in a way that aligns better with the modern patient’s lifestyle.
The Problem with Pills: Why Oral Sildenafil Has Reached Its Limitations
Oral sildenafil is effective, but its pharmacokinetics are often inconveniently unpredictable. Absorption through the gastrointestinal tract can be slowed by food intake, especially fatty meals, leading to inconsistent plasma concentrations and reduced efficacy. Furthermore, hepatic metabolism via CYP3A4 reduces systemic availability to about 40%, meaning much of the drug never reaches its target tissues.
This variability translates into real-world frustrations. Many patients report that the timing of the drug is difficult to synchronize with sexual activity, undermining spontaneity and satisfaction. For others—particularly older men with hepatic or renal impairment—the systemic exposure to sildenafil can increase the risk of hypotension or headache, discouraging long-term use.
From a clinical standpoint, the goal is clear: maintain the therapeutic benefits of PDE5 inhibition while minimizing systemic peaks and side effects. A non-oral route capable of steady, controlled delivery offers exactly that—transdermal therapy.
Transdermal Delivery: A Clinically Logical Alternative
The skin is an attractive, though challenging, route for systemic drug delivery. It provides a large, accessible surface area, avoids gastrointestinal degradation, and bypasses hepatic first-pass metabolism. For lipophilic and low-molecular-weight drugs like sildenafil, transdermal absorption is theoretically achievable—but practically hindered by the stratum corneum, the skin’s formidable barrier.
Traditional transdermal patches have limited success with sildenafil due to its moderate solubility and molecular rigidity. The innovation described in this study overcomes that limitation using transfersomes—ultra-deformable lipid vesicles designed to penetrate through skin microchannels without disrupting the barrier.
In clinical terms, this means that instead of relying on hepatic metabolism and variable intestinal absorption, sildenafil could be absorbed directly into systemic circulation through the dermis. The potential benefits include:
- More stable plasma levels, avoiding sharp peaks and troughs;
- Lower required doses, reducing side effects;
- Enhanced patient adherence, thanks to convenience and comfort.
The technology effectively merges pharmaceutical sophistication with clinical practicality—precisely what physicians need to translate scientific innovation into therapeutic impact.
Transfersomes: How They Work and Why They Matter Clinically
Transfersomes are not ordinary liposomes. They are ultra-flexible lipid vesicles composed of phospholipids and edge activators such as surfactants, which disrupt lipid packing and confer remarkable deformability. This allows them to squeeze through skin pores far smaller than their own diameter while maintaining structural integrity.
Once applied to the skin, these vesicles migrate through the intercellular lipid pathways of the stratum corneum, driven by the skin’s natural hydration gradient. In effect, the transfersomes act like molecular “shuttles,” carrying sildenafil into deeper layers of the skin and then into systemic circulation.
For clinicians, this mechanism translates into enhanced delivery efficiency and prolonged systemic availability. In animal models, the sildenafil-loaded transfersomal hydrogel achieved more than double the bioavailability compared to oral tablets. Plasma concentrations were maintained longer, suggesting potential for once-daily or even every-other-day dosing, depending on patient response and formulation optimization.
Equally important, the system allows for localized drug reservoirs in the dermal layers, ensuring a steady diffusion into circulation without the gastrointestinal irritation or blood pressure fluctuations seen with oral therapy.
The Hydrogel Advantage: Stability, Comfort, and Clinical Usability
Embedding the transfersomes in a hydrogel matrix enhances both patient comfort and pharmacologic control. Hydrogels, typically composed of biocompatible polymers like carbopol or hydroxyethyl cellulose, provide a soft, cooling, and non-greasy base ideal for dermal application.
From a pharmaceutical perspective, the hydrogel serves several key roles:
- Stabilization of transfersomal vesicles by preventing aggregation or fusion;
- Controlled release through gradual hydration and diffusion;
- Improved adherence to skin, ensuring consistent contact time for effective absorption.
Clinically, this means the patient experiences a transparent, easy-to-apply gel that absorbs quickly without residue or odor—qualities that strongly influence adherence in long-term treatments. In contrast to transdermal patches, the hydrogel can be discreetly applied, adjusted for dose flexibility, and removed effortlessly.
In the in vivo studies, the formulation demonstrated sustained plasma sildenafil concentrations for over 24 hours, with no skin irritation or sensitization observed—a reassuring safety profile for potential clinical use.
Pharmacokinetic and Therapeutic Implications
The pharmacokinetic performance of the transfersomal hydrogel formulation represents a major advance. Compared with oral sildenafil, the transdermal system produced higher area-under-the-curve (AUC) values, indicating improved systemic exposure. The maximum plasma concentration (Cmax) was reached more gradually, minimizing the abrupt peaks responsible for vasodilatory side effects.
For patients, this could translate into a more natural and sustained erectile response, aligning drug action with the physiological rhythm of sexual activity rather than a narrow time window. Furthermore, the extended-release profile reduces the need for strict timing—a common psychological barrier to spontaneous intimacy.
From a physician’s perspective, these pharmacokinetic benefits may expand the drug’s use beyond ED to conditions where chronic sildenafil therapy is beneficial, such as pulmonary arterial hypertension (PAH) or Raynaud’s phenomenon. Transdermal administration would mitigate systemic side effects and provide more stable plasma levels for continuous vascular modulation.
Safety Profile: Reducing Systemic and Local Adverse Effects
One of the most compelling aspects of this innovation is its safety. The localized, controlled release of sildenafil minimizes the sudden hemodynamic shifts that can occur with oral administration. In preclinical models, no significant changes in blood pressure or heart rate were observed, even at therapeutic-equivalent doses.
Additionally, skin tolerance was excellent. Histopathological examination of treated skin revealed no signs of inflammation, necrosis, or barrier disruption. The use of natural phospholipids and mild surfactants ensures the formulation remains dermatologically safe, even with repeated use.
For clinicians managing patients with cardiovascular comorbidities—who may be at higher risk from systemic vasodilators—this delivery system offers a safer pharmacodynamic profile, potentially expanding the eligible population for sildenafil therapy.
Clinical Translation: Who Stands to Benefit?
Transdermal sildenafil may particularly benefit several patient populations currently underserved by oral therapy:
- Elderly patients with reduced hepatic function, in whom first-pass metabolism diminishes oral bioavailability;
- Men with gastrointestinal disorders (e.g., peptic ulcer, malabsorption syndromes) that interfere with drug absorption;
- Patients on polypharmacy, where CYP3A4 interactions (e.g., with antifungals or antihypertensives) complicate dosing;
- Patients with dysphagia or aversion to tablets, for whom a topical alternative improves compliance.
Furthermore, the system could improve adherence among patients with mild-to-moderate ED, who often discontinue oral therapy due to side effects or inconsistent timing. A once-daily topical regimen, applied discreetly and comfortably, aligns better with patient lifestyle and expectations.
A Practical Perspective: Integration into Clinical Practice
For clinicians, adopting transdermal sildenafil in practice will require minimal adaptation. The hydrogel could be prescribed similarly to other topical treatments—applied to clean, hairless skin areas such as the upper arm or abdomen. Dosing could be titrated according to response, and plasma sildenafil levels would remain stable over several hours, allowing flexibility in sexual activity timing.
Such an approach could integrate seamlessly into multimodal management of erectile dysfunction, complementing psychosexual counseling, cardiovascular risk reduction, and lifestyle modification. Moreover, because the hydrogel avoids hepatic metabolism, it may reduce the need for dose adjustments in patients with hepatic impairment.
Importantly, clinicians should counsel patients on proper application technique, emphasizing even distribution, avoidance of occlusion, and skin hygiene. As clinical trials advance, standardized dosing recommendations will likely emerge, simplifying prescription patterns.
The Broader Vision: Beyond Erectile Dysfunction
While developed primarily for ED, this technology holds promise across multiple vascular and dermatologic domains. Sildenafil’s vasodilatory and cytoprotective effects make it a candidate for cutaneous wound healing, pressure ulcer prevention, and even ischemic skin disorders.
The transfersomal hydrogel system could also serve as a template for other PDE5 inhibitors—such as tadalafil or vardenafil—or for entirely different pharmacologic classes where transdermal delivery remains elusive. This represents an exciting convergence of pharmaceutical innovation and clinical pragmatism.
The broader implication is clear: if we can reengineer classic oral drugs into transdermal formulations, we can extend their benefits to populations previously limited by pharmacokinetics or side effects. This is personalized medicine, realized through material science.
Limitations and the Road to Human Application
Despite its promise, the transfersomal hydrogel remains in the preclinical phase, and several challenges must be addressed before clinical use.
- Scaling production while maintaining vesicle stability is complex, requiring stringent control of lipid composition and surfactant ratios.
- Long-term safety data are needed, particularly regarding repeated application and potential accumulation in dermal layers.
- Interpatient variability in skin permeability—affected by age, hydration, and anatomical site—must be studied to optimize dosing.
Nevertheless, these are surmountable barriers. Similar nanocarrier-based systems (for insulin, lidocaine, and hormones) have already reached the clinic, proving that regulatory and manufacturing pathways are viable. With sildenafil’s established safety record, clinical translation could progress rapidly once pharmacokinetic equivalence is demonstrated in humans.
Conclusion: A Smarter Route for a Familiar Drug
The development of sildenafil-loaded transfersomal hydrogel exemplifies the future of rational pharmacotherapy: using advanced delivery systems to refine—not reinvent—effective drugs. For clinicians, this represents a practical and potentially transformative option for patients struggling with the limitations of oral sildenafil.
By achieving enhanced bioavailability, prolonged action, and improved tolerability, this formulation reimagines what ED treatment can look like—convenient, discreet, and physiologically aligned with patient needs. As clinical trials advance, transdermal sildenafil may soon redefine the standard of care, marking a shift from “taking a pill” to wearing therapy.
FAQ: Transdermal Sildenafil in Clinical Practice
1. How does the transdermal sildenafil hydrogel compare in efficacy to oral tablets?
Early studies suggest equivalent or superior bioavailability, with a slower but steadier systemic absorption profile. This may translate to more predictable efficacy and fewer side effects.
2. Are there any risks of skin irritation or systemic hypotension?
Preclinical data show excellent skin tolerance and no significant cardiovascular effects. However, clinical trials are still required to confirm safety in diverse patient populations.
3. When might this formulation become available clinically?
Given the encouraging preclinical results, early human studies could begin within the next few years. If successful, a market-ready product might appear within five to seven years.