Preterm birth remains one of the greatest unresolved challenges in modern obstetrics. Despite decades of pharmacological innovation, the global incidence of premature birth exceeds 15 million cases annually, with neonatal morbidity and mortality remaining unacceptably high. Conventional tocolytics—β₂-agonists, calcium channel blockers, magnesium sulfate, prostaglandin inhibitors, and nitric oxide donors—offer transient relief but often at the cost of maternal cardiovascular or metabolic side effects.
A recent experimental study by Barna et al. (2023, University of Szeged, Hungary) brings new insight into this problem by examining a novel combination therapy: sildenafil citrate, a phosphodiesterase type 5 (PDE5) inhibitor, and terbutaline, a classic β₂-adrenergic agonist. Their findings, although based on animal models, open the door to a safer, more effective approach to uterine relaxation and potentially, preterm labor prevention.
Understanding the Physiology of Uterine Contraction and Relaxation
The myometrium is not a simple mechanical organ. It is a finely tuned network of smooth muscle fibers regulated by hormonal, neural, and molecular signals. Under normal pregnancy conditions, the uterus maintains quiescence through a balance of cyclic nucleotides—cyclic AMP (cAMP) and cyclic GMP (cGMP)—which mediate relaxation pathways.
During preterm labor, this balance is disrupted. Pro-inflammatory cytokines, stretch-induced signaling, and reduced nitric oxide (NO) bioavailability all converge to increase intracellular calcium, stimulating myosin light-chain kinase and promoting contraction. Consequently, tocolytic therapy focuses on restoring intracellular signaling pathways that favor relaxation.
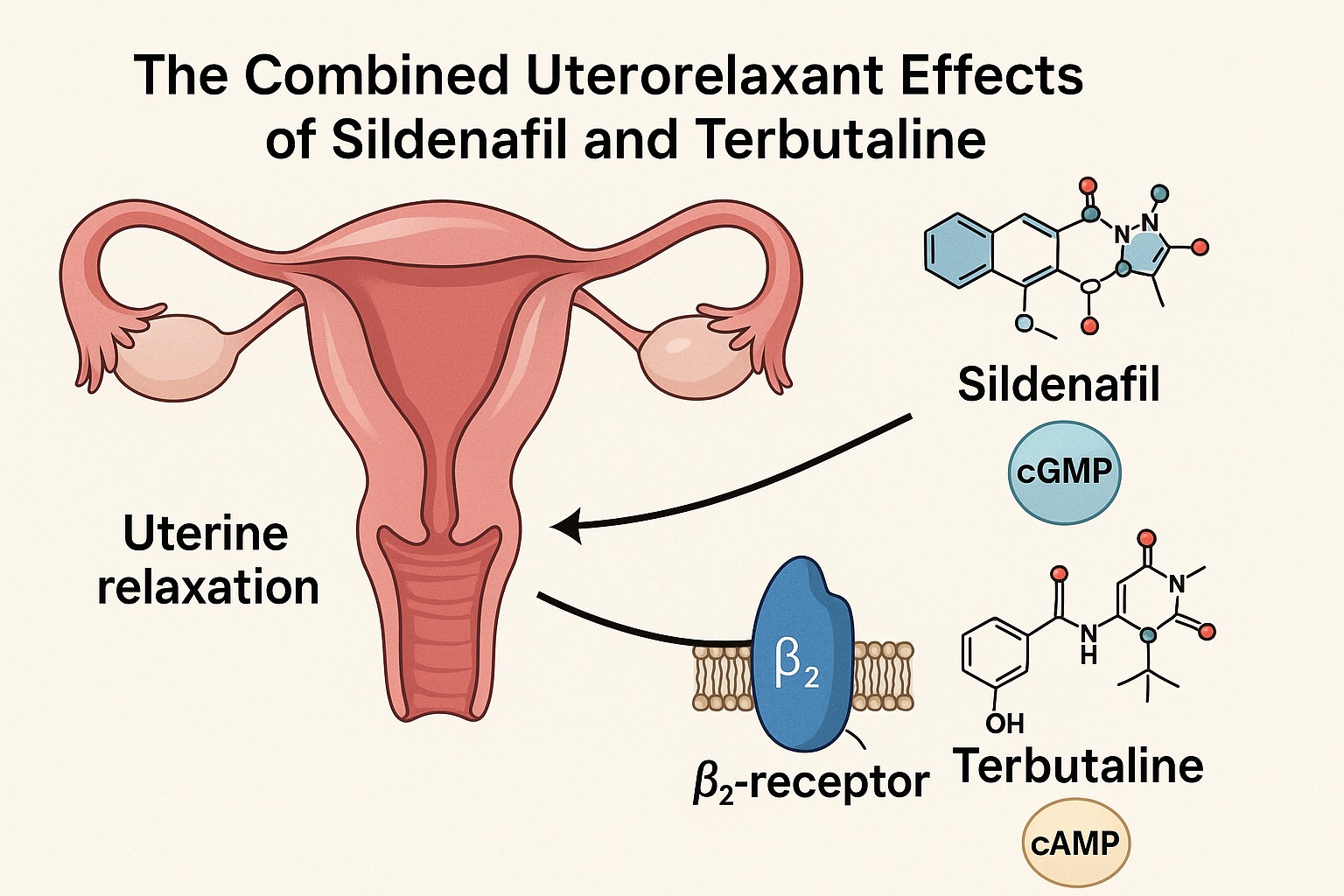
β₂-agonists like terbutaline increase cAMP levels via adenylate cyclase activation, inhibiting myosin phosphorylation and reducing contractility. Meanwhile, PDE5 inhibitors such as sildenafil prevent cGMP degradation, prolonging NO-mediated relaxation. By targeting complementary pathways, their combination holds theoretical promise for additive—or even synergistic—uterine relaxation with lower doses and fewer adverse effects.
The Rationale Behind Combining Sildenafil and Terbutaline
Monotherapy with either β₂-agonists or PDE5 inhibitors presents clinical limitations. Terbutaline, though effective, is often curtailed by maternal tachycardia, palpitations, tremor, and pulmonary edema, particularly when administered at higher doses. Conversely, sildenafil’s hemodynamic effects can induce hypotension or mild headache, and its obstetric use remains largely experimental.
The central hypothesis explored in this research is elegantly simple: could combining both drugs at subtherapeutic doses produce sufficient uterine relaxation while minimizing systemic side effects?
The theoretical synergy is rooted in intracellular signaling. Terbutaline boosts cAMP, and sildenafil prevents cGMP breakdown—both pathways ultimately decrease cytoplasmic calcium levels, enhancing smooth muscle relaxation. Additionally, terbutaline may indirectly potentiate the NO–cGMP pathway, amplifying sildenafil’s efficacy. Thus, the combination has the potential to offer strong tocolysis without cardiovascular compromise, a long-sought goal in obstetric pharmacotherapy.
Methodological Framework: From Organ Bath to In Vivo Validation
The study employed a robust dual-approach design combining in vitro and in vivo methods in pregnant rats at various gestational stages (days 5–22).
In the organ bath experiments, uterine rings were isolated and exposed to increasing concentrations of sildenafil or terbutaline individually, and then in combination. Muscle tension was continuously measured to assess dose-dependent effects on spontaneous and KCl-induced contractions. The researchers calculated EC₅₀ and Emax values to quantify efficacy and potency.
To complement these findings, in vivo electromyographic (SMEMG) recordings were conducted on anesthetized, 22-day pregnant rats. This approach provided a dynamic measure of myometrial activity, representing the physiological context more closely. Additionally, biochemical assays were performed to quantify cAMP and cGMP levels, elucidating the intracellular mechanisms behind observed uterorelaxant effects.
This design provided a comprehensive perspective: molecular, functional, and systemic insights converged to clarify how each drug—and their combination—acts on uterine smooth muscle.
Sildenafil Alone: Modest but Reliable Uterine Relaxation
As a selective PDE5 inhibitor, sildenafil primarily prolongs the action of nitric oxide by preventing the breakdown of cGMP, leading to vasodilation and smooth muscle relaxation. The study confirmed that sildenafil induces a dose-dependent inhibition of uterine contractions across all gestational stages.
Interestingly, its maximal efficacy was observed during early and mid-pregnancy, decreasing slightly toward term (gestational day 22). This reduction is likely due to increased NO metabolism and altered receptor sensitivity near labor onset. Despite this, sildenafil maintained a robust relaxant effect—over 80% inhibition of uterine activity at high concentrations.
From a clinical standpoint, this suggests that sildenafil could serve as a foundation for new tocolytic strategies, particularly in women at risk for early preterm labor or with uteroplacental perfusion defects. Its safety profile and established pharmacokinetics in pregnancy (through studies on fetal growth restriction) make it an attractive candidate for further exploration.
Terbutaline: The Classic β₂-Agonist Revisited
Terbutaline remains a cornerstone β₂-adrenergic agonist with powerful uterine relaxation properties. Acting through adenylate cyclase activation, it raises intracellular cAMP, suppressing calcium-mediated myometrial contraction.
However, decades of clinical experience have shown that while terbutaline is effective for acute tocolysis, its chronic use offers little benefit and carries significant risks. Side effects such as tachycardia, hyperglycemia, and pulmonary edema have led to its withdrawal from long-term use in many countries.
Nevertheless, the drug’s mechanism remains potent. When used judiciously—especially at lower doses in combination therapy—terbutaline may reclaim a role in modern obstetric pharmacology. The study’s findings suggest that adding terbutaline at submaximal concentrations enhances sildenafil’s effect at lower doses, implying that the combination can achieve relaxation thresholds safely unattainable with either drug alone.
The Biochemical Signature: cAMP, cGMP, and Dual Pathway Enhancement
One of the study’s key strengths lies in its exploration of intracellular signaling mechanisms. Measurements of uterine cAMP and cGMP levels revealed that sildenafil primarily elevated cGMP in a concentration-dependent manner, whereas terbutaline alone did not significantly alter cGMP levels but increased cAMP.
When co-administered, terbutaline enhanced sildenafil-induced cGMP elevation, particularly at lower sildenafil concentrations, without significantly modifying cAMP. This finding is crucial: it indicates that the synergistic uterorelaxation seen in vivo may not stem from dual cyclic nucleotide accumulation, but rather from terbutaline’s indirect stimulation of the NO–cGMP pathway.
Clinically, this points toward a potentially energy-efficient molecular synergy, where low doses of each agent achieve maximal relaxation without receptor desensitization or cardiovascular compromise. Such pharmacodynamic harmony could redefine how obstetricians approach tocolytic combinations.
Translating Animal Data to Human Obstetrics: Promise and Precautions
While the results are compelling, the translation from rat models to human pregnancy warrants caution. The rat uterus differs in structure, hormonal milieu, and receptor distribution from the human myometrium. However, previous studies in human tissue samples have already shown that sildenafil can relax the pregnant myometrium, supporting potential cross-species applicability.
The clinical significance of these findings lies in dose modulation. The study demonstrated that adding low-dose terbutaline reduced the ED₅₀ of sildenafil nearly threefold, meaning smaller doses could achieve equivalent uterine relaxation. This reduction in required dosage could dramatically lower maternal side effect risk, an enduring challenge in tocolytic therapy.
For obstetric practice, this translates into a possible personalized tocolysis model: using existing, well-characterized drugs in carefully balanced combinations, guided by pharmacodynamic insights rather than empirical trial-and-error. It is a step toward mechanism-driven obstetric pharmacology.
Clinical Implications: Rethinking Tocolytic Strategy
If validated in human trials, the combination of sildenafil and terbutaline could shift current management paradigms for preterm labor. The potential advantages are multifold:
- Enhanced efficacy at lower doses, reducing side effects such as maternal tachycardia or hypotension.
- Improved fetal safety, avoiding excessive uteroplacental vasodilation or fetal tachyarrhythmias.
- Mechanistic complementarity, as both drugs act on distinct but converging pathways to achieve uterine quiescence.
Moreover, both agents possess anti-inflammatory properties, which may prove beneficial in infection-related or inflammatory preterm labor, a common yet poorly addressed cause of early contractions. The dual anti-inflammatory and smooth muscle–relaxant actions make this combination particularly promising for multifactorial preterm birth syndromes.
From a pragmatic standpoint, clinicians should remain aware that while these results are promising, pharmacovigilance and careful dosing remain essential. Low-dose combinations could become a next-generation tocolytic strategy, provided future studies confirm safety and efficacy in humans.
Limitations and Future Directions
Every translational step from bench to bedside must acknowledge its limitations. This study’s conclusions, though robust within the experimental framework, arise from rodent models that cannot fully replicate human physiology.
No direct data on maternal cardiovascular or fetal heart rate effects were collected, leaving open questions about safety margins. Additionally, the pharmacokinetics of co-administered sildenafil and terbutaline in human pregnancy remain unknown.
Future research must focus on:
- Controlled clinical trials assessing efficacy, tolerability, and fetal outcomes.
- Exploration of alternative β₂-agonists or PDE5 inhibitors with optimized selectivity profiles.
- Investigation of NO-independent mechanisms, particularly in cases where nitric oxide signaling is impaired (e.g., preeclampsia).
If these areas are addressed, this line of research could yield one of the most meaningful advances in obstetric pharmacology in decades.
Conclusion: Toward a New Era of Rational Tocolysis
The combined administration of sildenafil and terbutaline represents an exciting and scientifically sound innovation in the field of tocolysis. Acting through distinct yet complementary cyclic nucleotide pathways, the two drugs demonstrated a synergistic uterine relaxant effect in pregnant rats, particularly at lower doses.
Clinically, this suggests the potential for safer, more targeted interventions in the prevention and management of preterm labor. By integrating modern pharmacological insight with established therapeutic agents, we may finally approach a model of precision obstetric medicine, where efficacy is maximized and risk minimized.
While further human research is essential, the concept itself—a low-dose, mechanism-based combination therapy—marks an important step toward achieving the long-sought goal of effective and safe uterine relaxation.
FAQ: Sildenafil and Terbutaline in Preterm Labor
1. Can sildenafil and terbutaline really work together to prevent preterm labor in humans?
Preliminary animal data suggest strong potential synergy, with enhanced uterine relaxation at low doses. However, clinical validation in pregnant women is still required before this combination can be recommended.
2. Why use low doses instead of standard tocolytic doses?
Lower doses reduce the risk of cardiovascular and metabolic side effects—such as hypotension or tachycardia—while maintaining effectiveness through synergistic molecular signaling.
3. Is sildenafil safe to use during pregnancy?
Sildenafil has been used experimentally for conditions like fetal growth restriction and preeclampsia. While generally well-tolerated, its use as a tocolytic remains investigational pending further safety and efficacy studies.