Introduction
The human brain is an organ of remarkable resilience, yet it is also exquisitely sensitive to the wear and tear of life’s stressors. Modern life demands multitasking, constant availability, and relentless performance, all of which place the central nervous system under chronic psychosocial pressure. While a brief surge of stress hormones may sharpen attention, prolonged exposure can chip away at memory and learning. At the molecular level, stress reshapes synapses, suppresses trophic support, and undermines neuronal vitality.
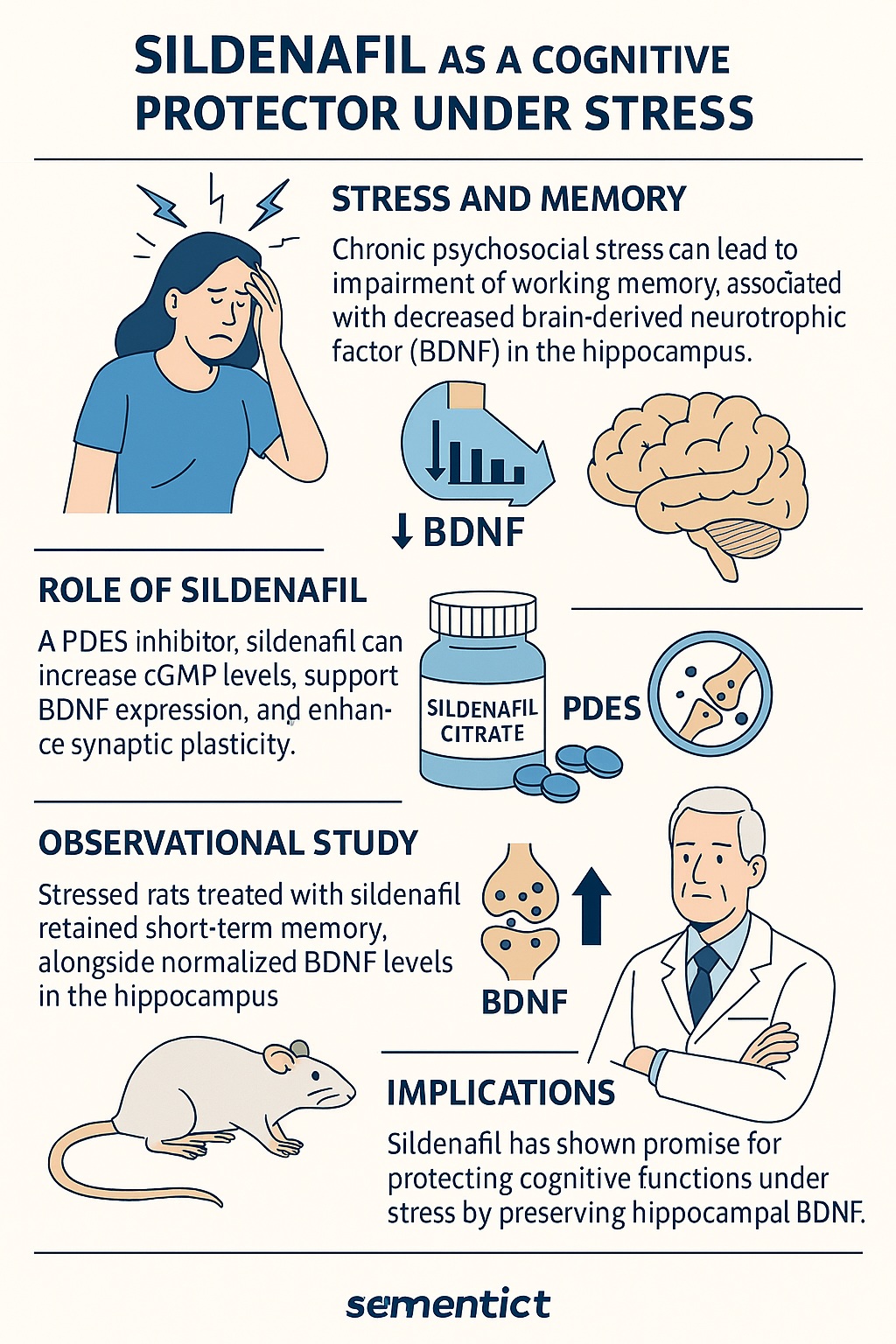
One molecule repeatedly implicated in this decline is brain-derived neurotrophic factor (BDNF). Often described as a molecular fertilizer for neurons, BDNF sustains synaptic plasticity, strengthens circuits, and safeguards cognitive performance. Unfortunately, psychosocial stress reduces hippocampal BDNF expression, correlating with impaired short-term memory. The result: a brain less capable of storing and retrieving information when it is most needed.
Intriguingly, a pharmacological agent initially designed for a very different indication—sildenafil citrate, the well-known treatment for erectile dysfunction—has demonstrated a protective role in preserving memory under stress. By inhibiting phosphodiesterase type 5 (PDE5), sildenafil amplifies cyclic guanosine monophosphate (cGMP) signaling, which in turn supports synaptic plasticity. Recent research suggests that this mechanism also intersects with BDNF regulation, offering a potential lifeline to stressed neurons.
This article explores the findings of recent experimental work on sildenafil’s neuroprotective effects in the context of chronic psychosocial stress, examines the central role of BDNF, and reflects on the broader implications for neuroscience, pharmacology, and even psychiatry.
Stress and the Fragile Architecture of Memory
To appreciate sildenafil’s unexpected benefits, one must first understand how stress sabotages cognition. Stress is not inherently pathological; in fact, acute stress can enhance memory consolidation by activating glucocorticoids and catecholamines. Yet chronic, unrelenting stress tips the balance toward harm.
At the hippocampal level, stress drives glucocorticoid receptor overactivation and elevates excitatory amino acid release, particularly glutamate. Excess glutamate leads to excitotoxicity and dendritic atrophy. Structural remodeling of hippocampal neurons reduces synaptic density, creating a less efficient substrate for memory formation.
Crucially, chronic psychosocial stress consistently lowers BDNF expression. This neurotrophin is essential for long-term potentiation (LTP), the electrophysiological process underlying learning. A deficit in BDNF translates into impaired synaptic plasticity and diminished working memory performance. Behavioral studies in both animals and humans confirm that stressed subjects show reduced ability to recall newly learned tasks, particularly those relying on the hippocampus.
The clinical implications are sobering. Chronic stress has been linked not only to cognitive impairment but also to major depressive disorder, post-traumatic stress disorder, and accelerated neurodegeneration. Thus, strategies to protect hippocampal integrity during stress exposure are of urgent interest.
Sildenafil: Beyond Its Vascular Role
Sildenafil citrate rose to fame as the first oral therapy for erectile dysfunction, thanks to its selective inhibition of PDE5 in the corpus cavernosum. By blocking PDE5, sildenafil prevents the breakdown of cGMP, leading to smooth muscle relaxation and enhanced blood flow.
What is less widely appreciated is that PDE5 is also expressed in the central nervous system, particularly in brain regions critical for cognition, including the hippocampus and cortex. Within neurons, cGMP acts as a secondary messenger, modulating synaptic plasticity and memory processes. Animal studies have shown that increasing cGMP can improve object recognition, spatial learning, and even memory consolidation after brain insults.
Sildenafil’s pharmacological footprint extends further: it has antioxidant properties, reduces excitotoxicity, and modulates nitric oxide signaling—all pathways relevant to stress-induced neurotoxicity. Together, these effects create a strong rationale for investigating sildenafil as a potential cognitive protector.
The Experimental Evidence: Stress, Memory, and Sildenafil
Recent experimental work using male Wistar rats provides compelling insights into sildenafil’s role under conditions of chronic psychosocial stress. Stress was induced through an intruder paradigm, a well-established model that forces rats to continuously renegotiate social hierarchies, thereby mimicking the unpredictability and interpersonal tension of human stress.
Animals were divided into four groups:
- Control (no stress, no drug)
- Stress only
- Sildenafil only
- Stress plus sildenafil
Sildenafil was administered intraperitoneally at a dose of 3 mg/kg/day for two months. Behavioral assessment relied on the radial arm water maze (RAWM), a test of spatial learning and both short- and long-term memory.
The results were striking.
- Stressed rats demonstrated short-term memory impairment—making significantly more errors during recall at 30 minutes.
- Long-term memory at 5 and 24 hours remained largely intact.
- Rats receiving sildenafil alongside stress exposure retained normal short-term memory performance, indistinguishable from controls.
- Importantly, sildenafil alone did not enhance memory in unstressed animals, suggesting its role is protective rather than enhancing in the absence of pathology.
At the molecular level, stress reduced hippocampal BDNF expression. Sildenafil prevented this decline, effectively normalizing BDNF levels. Oxidative stress markers, however, showed minimal alteration across groups, with the exception of oxidized glutathione levels, which were elevated under stress but mitigated by sildenafil.
These findings converge on a clear message: sildenafil shields short-term memory under stress, likely through preservation of hippocampal BDNF, rather than broad antioxidant effects.
Brain-Derived Neurotrophic Factor: The Molecular Pivot
Why does BDNF occupy center stage in this narrative? Simply put, without adequate BDNF, neurons struggle to adapt, grow, and encode experiences. BDNF binds to the TrkB receptor, triggering intracellular cascades that promote dendritic spine formation, synaptic plasticity, and survival pathways.
Under chronic stress, glucocorticoids suppress BDNF transcription, while excitotoxic signaling accelerates neuronal wear. The hippocampus, richly endowed with glucocorticoid receptors, is particularly vulnerable. Declining BDNF levels correlate with reduced long-term potentiation, structural atrophy, and impaired working memory.
Sildenafil appears to restore BDNF balance. By increasing cGMP levels, sildenafil may indirectly promote BDNF transcription and stabilize synaptic networks. This aligns with earlier findings in disease models such as Alzheimer’s disease and diabetes, where sildenafil normalized BDNF deficits and improved cognitive outcomes.
The protective effect is therefore not a generalized cognitive booster but a stress-contingent safeguard, activating only when the hippocampus is threatened.
Oxidative Stress: A More Modest Role
One might expect oxidative stress to dominate the discussion, as it often does in neurodegeneration. Reactive oxygen species damage lipids, proteins, and DNA, undermining cellular homeostasis. In fact, acute stress reliably increases oxidative markers, and antioxidants can rescue learning deficits.
Surprisingly, in the intruder stress paradigm, oxidative stress biomarkers (SOD, catalase, GPx, TBARS, reduced glutathione) showed no significant differences between groups, except for oxidized glutathione. This suggests that not all stress paradigms elicit the same oxidative profile, and duration, type, and intensity of stress matter profoundly.
Thus, while sildenafil has demonstrated antioxidant effects in other contexts, its protective role here appears only marginally linked to oxidative pathways. Instead, the preservation of BDNF emerges as the dominant mechanism.
Implications for Human Health
Translating rodent findings to humans requires caution, but the implications are tantalizing. If sildenafil can protect hippocampal-dependent memory under stress conditions, potential applications extend far beyond its urological use.
Possible clinical avenues include:
- Stress-related cognitive impairment: Individuals in high-pressure professions may benefit from pharmacological safeguards against working memory erosion.
- Neuropsychiatric disorders: Depression and PTSD involve reduced BDNF levels and hippocampal dysfunction. PDE5 inhibition could complement existing therapies.
- Neurodegenerative conditions: Alzheimer’s disease and vascular cognitive impairment feature both BDNF decline and synaptic failure. Sildenafil’s role as a synaptic stabilizer deserves further exploration.
That said, enthusiasm must be tempered. The current evidence is preclinical, restricted to male animals, and based on controlled stress paradigms. Clinical trials in diverse human populations are essential before prescribing sildenafil as a cognitive shield. Safety, dosage, and long-term effects must all be carefully evaluated.
Limitations and Future Directions
The study offers robust insights but is not without limitations. Only male rats were included, leaving sex-specific differences unexplored. Female brains, influenced by estrogen and its interaction with BDNF, may respond differently. Furthermore, the stress model captures only one dimension of psychosocial strain. Other forms—restraint, isolation, or trauma—might yield divergent results.
Molecular analysis was focused on BDNF and select oxidative markers. Yet stress undoubtedly influences other signaling pathways: inflammatory cytokines, synaptic scaffolding proteins, and epigenetic regulators, to name a few. Integrating these layers would paint a fuller picture of sildenafil’s actions.
Finally, the absence of cognitive improvement in unstressed rats is both reassuring and limiting. While it underscores sildenafil’s safety in not overstimulating normal brains, it raises questions about whether its benefits are restricted only to pathological conditions.
Conclusion
The story of sildenafil and cognition is a testament to pharmacology’s serendipity. A drug developed to enhance blood flow has revealed a second life as a protector of stressed synapses. By preventing the decline of hippocampal BDNF, sildenafil shields working memory from the erosive effects of chronic psychosocial stress.
This finding not only enriches our understanding of stress biology but also challenges us to reconsider how existing drugs may be repurposed. As research progresses, the possibility of prescribing sildenafil—or related PDE5 inhibitors—for cognitive resilience may move from laboratory speculation to clinical reality. Until then, the lesson is clear: nurturing synaptic plasticity, whether by lifestyle, therapy, or pharmacology, remains at the heart of preserving memory in a stressful world.
FAQ
1. Does sildenafil improve memory in healthy individuals?
No. Evidence suggests sildenafil does not enhance learning or memory in unstressed, healthy brains. Its protective effect emerges only under conditions of stress or pathology where BDNF levels decline.
2. How does sildenafil influence brain-derived neurotrophic factor (BDNF)?
By inhibiting PDE5 and elevating cGMP signaling, sildenafil indirectly supports BDNF expression in the hippocampus. This helps maintain synaptic plasticity and prevents short-term memory impairment during stress.
3. Could sildenafil become a treatment for stress-related disorders in humans?
Potentially, yes. Preclinical data are promising, especially for conditions involving hippocampal dysfunction and reduced BDNF. However, clinical trials in humans are needed to determine efficacy, safety, and appropriate dosing.