Introduction
When sildenafil citrate—better known by its trade name Viagra—entered the market in the late 1990s, it did more than transform the management of erectile dysfunction. It altered conversations around sexuality, aging, and male reproductive health. Almost immediately, clinicians, researchers, and even couples hoping for conception began asking an obvious but underexplored question: does sildenafil influence fertility?
The answer matters. Erectile dysfunction and infertility frequently coexist, often rooted in overlapping conditions such as diabetes, vascular disease, or psychological stress. A drug that restores erections but compromises sperm quality would be a cruel paradox. Conversely, if sildenafil improves sperm function, it could become more than a facilitator of intercourse—it could directly enhance conception rates.
The study at hand, a randomized, double-blind, placebo-controlled crossover trial involving 20 healthy men, sought to answer this question with unusual rigor. It tested the effects of both sildenafil and a cGMP analog (8-bromo-cGMP) on semen quality and sperm function. Its findings were illuminating: while sildenafil left most semen parameters unchanged, it significantly improved sperm motility and zona pellucida binding, suggesting potential reproductive benefits without adverse consequences. This article unpacks these results, explores their biological plausibility, and considers their implications for clinical practice.
Erectile Dysfunction, Sildenafil, and Reproductive Questions
Erectile dysfunction (ED) is not just a barrier to intimacy—it is a major obstacle to reproduction. Couples trying to conceive may find their efforts derailed by the inability to achieve or maintain erections. For such couples, sildenafil is often a lifesaver, restoring confidence and sexual performance. But this raises a subtler issue: if a man can now achieve intercourse thanks to sildenafil, what is the drug doing at the cellular level to his sperm?
Historically, fertility research focused more on sperm concentration, morphology, and total semen volume than on pharmacological influences. Drugs with hormonal activity—such as anabolic steroids—are well known to suppress spermatogenesis. But PDE5 inhibitors like sildenafil act through vascular and smooth muscle pathways, not directly on the testis. Could they still affect sperm function?
The worry stems from the fact that sperm motility, capacitation, and acrosome reaction are all regulated by cyclic nucleotides—notably cyclic AMP (cAMP) and cyclic GMP (cGMP). Since sildenafil amplifies cGMP signaling by inhibiting its breakdown, it could theoretically alter sperm physiology. The study under discussion explored this very hypothesis, using both sildenafil and 8-bromo-cGMP to probe the system.
The Design: Crossover Precision
To move beyond speculation, the investigators designed a randomized, double-blind, placebo-controlled crossover study—the gold standard for assessing subtle drug effects. Twenty healthy men were recruited and randomized to receive either sildenafil or placebo. After a washout period, they crossed over to the alternate arm, ensuring that every participant served as his own control.
This design minimized inter-individual variability, an especially important feature when dealing with semen parameters, which are notoriously variable both between and within men. Samples were analyzed for standard semen quality metrics—volume, concentration, morphology—as well as functional assays such as motility, acrosome reaction, and zona pellucida binding. The inclusion of 8-bromo-cGMP added mechanistic depth by isolating the effects of enhanced cGMP signaling.
The methodological rigor ensured that observed changes could be confidently attributed to sildenafil rather than background fluctuations. For a topic often plagued by anecdote and assumption, this trial provided rare clarity.
The Findings: No Harm, Some Help
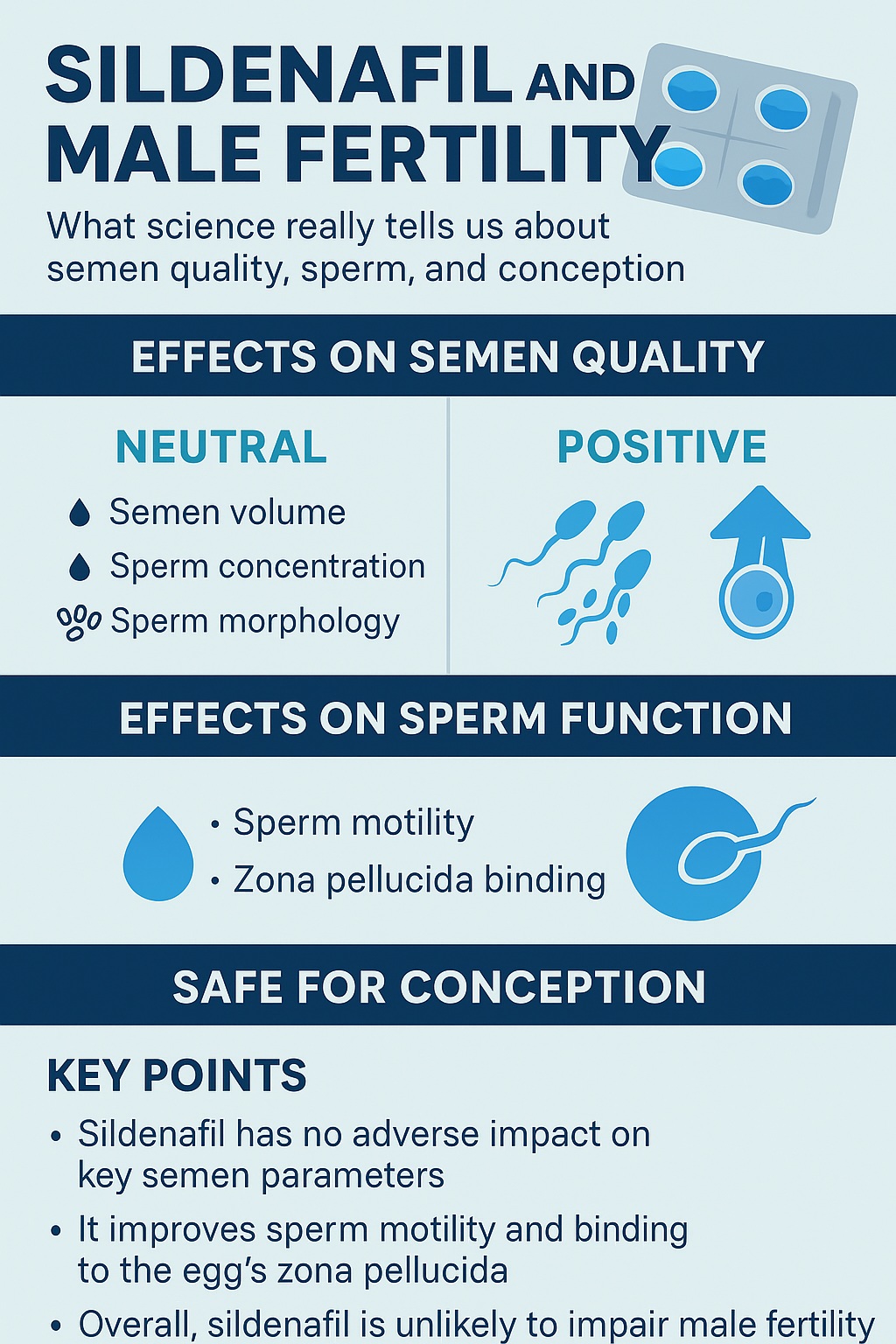
The study’s results can be summarized succinctly: sildenafil does not impair semen quality, and in some domains, it actually improves sperm function.
- No change in standard semen parameters: Semen volume, sperm concentration, and morphology were unaffected. This finding is critical, as it reassures clinicians and patients that sildenafil does not compromise the fundamental parameters of fertility.
- Improved motility: Sildenafil significantly increased the proportion of sperm exhibiting rapid, progressive motility—a parameter strongly correlated with fertilizing potential.
- Enhanced zona pellucida binding: The zona pellucida, a glycoprotein shell surrounding the oocyte, serves as a selective gateway for sperm. Sildenafil improved sperm’s ability to bind to this layer, suggesting enhanced competence for fertilization.
- No effect on acrosome reaction: The drug did not alter the acrosome reaction, a process critical for penetrating the oocyte but tightly regulated to occur only at the right moment.
In short, sildenafil left baseline semen quality untouched while boosting key aspects of functional fertility. For couples struggling to conceive, this dual reassurance—no harm, possible benefit—is highly relevant.
The Biology Behind the Results
Why would sildenafil improve motility and binding without affecting morphology or concentration? The answer lies in the biology of cyclic GMP signaling.
Sperm motility depends on the coordinated activity of flagellar dynein motors, fueled by ATP and modulated by intracellular signaling cascades. Cyclic nucleotides such as cAMP and cGMP serve as second messengers that fine-tune flagellar beating. By inhibiting PDE5, sildenafil prevents cGMP degradation, sustaining elevated levels within cells that respond to nitric oxide signaling.
In the vasculature, this leads to smooth muscle relaxation and vasodilation. In sperm, similar pathways may enhance flagellar activity, explaining the improved motility observed in the study. Importantly, sildenafil’s effect was not indiscriminate. It did not trigger premature acrosome reactions or alter morphology, suggesting that its influence is targeted and physiologically appropriate.
The improved zona pellucida binding likely reflects this enhanced motility and signaling competence. Binding is a multistep process requiring both mechanical vigor and biochemical readiness. By augmenting these properties, sildenafil primes sperm for successful fertilization without derailing their natural timing.
Clinical Implications: Beyond Erections
For clinicians, these findings carry both reassurance and opportunity. Reassurance, because sildenafil does not impair semen quality—a concern for men using the drug while trying to conceive. Opportunity, because enhanced motility and zona binding may increase the probability of natural conception, especially in couples where borderline sperm motility is a limiting factor.
This does not mean sildenafil should be prescribed as a fertility drug. Its primary indication remains erectile dysfunction, and its systemic effects—vasodilation, blood pressure changes, headaches—must not be trivialized. However, for men who already require sildenafil to achieve intercourse, the knowledge that it may also support sperm function is encouraging.
In assisted reproduction contexts, sildenafil may play an indirect role. By improving penile erection, it facilitates semen collection. By improving motility, it may slightly enhance in vitro fertilization outcomes. Yet these are adjunctive benefits, not primary indications. Overenthusiastic extrapolation risks medicalizing a drug beyond its remit.
The Cautionary Perspective
While the study is robust, caution remains necessary. The sample size—20 healthy men—was relatively small. Larger, more diverse populations, including men with subfertility, are needed to confirm and generalize the findings.
Additionally, the study evaluated short-term exposure. The effects of chronic sildenafil use on spermatogenesis remain less clear. Although PDE5 is not highly expressed in the testis, systemic influences—vascular, hormonal, metabolic—may emerge with long-term therapy. Current evidence suggests neutrality rather than harm, but surveillance is prudent.
Finally, fertility is more than semen analysis. It is the outcome of complex interactions between sperm, oocyte, reproductive tract, and time. Even if sildenafil enhances certain parameters, its ultimate impact on pregnancy rates must be evaluated through carefully designed clinical trials.
Integrating Evidence Into Practice
So how should clinicians integrate these findings into everyday practice? A few practical points emerge:
- Do not withhold sildenafil from men trying to conceive. The evidence strongly suggests no harm to semen or sperm function.
- Use sildenafil primarily for erectile dysfunction. Its reproductive benefits are incidental, not primary indications.
- Reassure patients with concerns. Men often worry that “chemicals” or “sexual aids” may damage fertility. Physicians can confidently explain that sildenafil is unlikely to pose such risks.
- Avoid off-label enthusiasm. Until larger studies confirm improved pregnancy rates, sildenafil should not be prescribed solely as a fertility booster.
This balanced approach ensures that patients receive the benefits of sexual function restoration without unnecessary fears or unrealistic expectations.
Broader Context: Fertility, Lifestyle, and Pharmacology
The sildenafil story also highlights a broader theme in reproductive medicine: the delicate balance between lifestyle, pharmacology, and fertility. Many couples assume infertility stems solely from female factors, overlooking the male contribution. Others assume pharmacological solutions can fix complex biological realities.
Sildenafil demonstrates that pharmacology can assist—but not replace—the fundamentals. Male fertility remains strongly influenced by age, weight, smoking, alcohol, and systemic disease. A pill that improves motility at the margins cannot counteract decades of vascular damage or metabolic dysfunction. In this sense, sildenafil is best viewed as an adjunct within a holistic fertility strategy, not a standalone solution.
Conclusion
The trial examining sildenafil’s impact on semen quality and sperm function offers an important message: sildenafil does not harm male fertility and may even enhance certain sperm functions. By improving motility and zona pellucida binding while leaving standard semen parameters unchanged, sildenafil reassures clinicians and patients alike that erectile restoration does not come at reproductive expense.
Yet, the drug should not be miscast as a fertility agent. Its primary role remains the treatment of erectile dysfunction. Any fertility benefits are secondary, supportive, and require further validation in larger studies. For couples navigating the twin challenges of infertility and erectile dysfunction, sildenafil offers a welcome paradox: a drug that helps achieve intercourse and may, at the same time, subtly improve the odds of conception.
FAQ
1. Does sildenafil harm sperm or semen quality?
No. Studies, including randomized controlled trials, show that sildenafil does not affect semen volume, sperm concentration, or morphology.
2. Can sildenafil improve fertility?
Indirectly, yes. Sildenafil improves sperm motility and zona pellucida binding, both important for fertilization. However, it should not be prescribed solely as a fertility drug.
3. Is long-term sildenafil use safe for men trying to conceive?
Current evidence suggests no harm, but long-term studies are limited. Men should use sildenafil under medical supervision, primarily for erectile dysfunction, while pursuing general health measures to optimize fertility.