Introduction
Modern oncology has shifted from blunt-force chemotherapeutics to targeted interventions that manipulate the tumor microenvironment (TME). At the heart of this paradigm is the recognition that cancer is not merely a collection of proliferating malignant cells but a dynamic ecosystem in which stromal cells, immune regulators, vasculature, and extracellular components collaborate—sometimes unwillingly—to sustain tumor survival. Among these cellular accomplices, myeloid-derived suppressor cells (MDSCs) have emerged as particularly insidious players.
First described in the context of chronic inflammation and malignancy, MDSCs are a heterogeneous population of immature myeloid cells that accumulate under pathological conditions. Their defining feature is not their surface markers—variable across species and contexts—but their potent immunosuppressive capacity. By inhibiting T cell proliferation, blunting natural killer (NK) cell activity, and fostering a tolerogenic milieu, MDSCs help tumors escape immune surveillance.
Over the past decade, attention has shifted from simply cataloguing MDSCs to actively targeting them as therapeutic obstacles. The rationale is straightforward: immune checkpoint inhibitors (ICIs) and adoptive cell therapies cannot succeed in an environment dominated by suppressor cells. Thus, modulating MDSCs—either by depleting them, inhibiting their recruitment, or reprogramming their function—has become an area of intense translational research. The story, however, is complicated. MDSCs are not monolithic, and strategies to tame them must navigate a fine line between tumor control and preserving essential host defense.
Origin and Biology of MDSCs
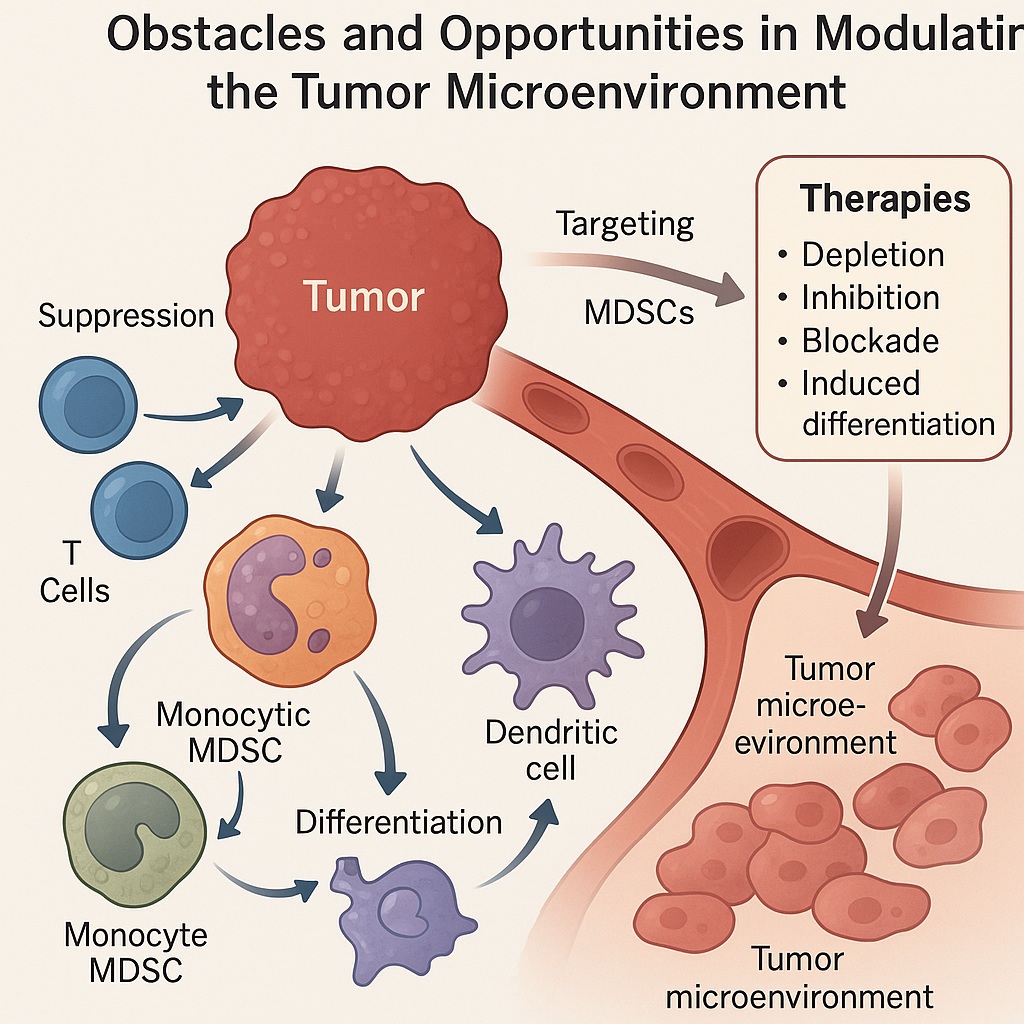
MDSCs arise from disrupted myelopoiesis. Under normal physiology, myeloid progenitors differentiate into granulocytes, macrophages, or dendritic cells. Chronic inflammation and tumor-derived factors derail this maturation, leading to accumulation of immature cells with suppressive functions. Two main subsets are typically described:
Polymorphonuclear MDSCs (PMN-MDSCs): Morphologically similar to neutrophils, abundant in most cancers.
Monocytic MDSCs (M-MDSCs): Resembling monocytes, with potent suppressive activity per cell.
These cells are recruited into the TME by chemokines such as CCL2, CXCL5, and CXCL12, and are expanded by growth factors like GM-CSF, VEGF, and G-CSF secreted by tumors. Once established in the tumor milieu, MDSCs unleash a variety of suppressive mechanisms.
Mechanistically, they deplete essential amino acids (arginine, tryptophan, cysteine) through enzymes such as arginase-1 and indoleamine 2,3-dioxygenase (IDO). They generate reactive oxygen and nitrogen species that impair T cell receptor signaling. They express checkpoint ligands such as PD-L1, directly silencing cytotoxic T cells. Beyond immunosuppression, MDSCs promote angiogenesis, remodel extracellular matrix, and support metastasis. In essence, they are the tumor’s loyal foot soldiers, defending malignancy against immune assault.
MDSCs and Resistance to Therapy
The relevance of MDSCs becomes most apparent when examining therapeutic resistance. Immune checkpoint inhibitors—anti-PD-1, anti-PD-L1, and anti-CTLA-4 antibodies—have revolutionized treatment for melanoma, lung cancer, renal carcinoma, and beyond. Yet, only a subset of patients experience durable responses. A recurring culprit in non-responders is the persistence of MDSCs.
Preclinical models consistently show that elevated MDSC infiltration correlates with reduced ICI efficacy. These cells establish an immunosuppressive barricade around tumors, neutralizing reinvigorated T cells. Similarly, MDSCs contribute to resistance against adoptive T cell transfer and cancer vaccines. Even traditional chemotherapeutics and radiotherapy, which can transiently reduce tumor burden, often paradoxically increase MDSC accumulation by inducing inflammatory cytokines.
The clinical data mirror these observations. High circulating or intratumoral MDSC counts predict poor prognosis in multiple cancers. For example, in non-small cell lung cancer, elevated PMN-MDSCs correlate with resistance to anti-PD-1 therapy. In breast cancer, M-MDSCs are linked to disease progression despite systemic therapy. These findings compel oncologists to consider not only the tumor itself but also its immunological entourage when tailoring treatment.
Strategies for Targeting MDSCs
Therapeutic approaches to MDSCs fall into several broad categories: depletion, functional inhibition, blockade of recruitment, and differentiation into less suppressive lineages. Each has advantages and limitations.
Depletion
Chemotherapeutic agents such as gemcitabine and 5-fluorouracil, at low doses, selectively reduce MDSC populations while sparing effector lymphocytes. Monoclonal antibodies targeting MDSC-associated surface molecules (e.g., CD33, CD11b) have also been investigated. However, systemic depletion risks collateral damage to normal myeloid cells, raising infection susceptibility.
Functional Inhibition
Drugs that block arginase, IDO, or nitric oxide synthase can blunt MDSC-mediated suppression. PDE5 inhibitors such as sildenafil, curiously better known for erectile dysfunction, reduce MDSC suppressive function in preclinical models, enhancing T cell activity. Other small molecules targeting STAT3, a master regulator of MDSC development, show promise in dampening their immunosuppressive arsenal.
Blockade of Recruitment
Interrupting chemokine signaling (e.g., CXCR2 or CCR2 antagonists) prevents MDSC trafficking into tumors. Such strategies reduce intratumoral MDSC density, allowing other immunotherapies to work more effectively.
Induced Differentiation
Agents like all-trans retinoic acid (ATRA) push MDSCs toward mature, non-suppressive myeloid fates. This not only reduces their suppressive function but also replenishes the immune system with competent antigen-presenting cells.
No single strategy is universally effective, and combination approaches often yield the best outcomes. The challenge remains tailoring these interventions to specific tumor contexts without tipping the balance toward systemic immunodeficiency.
Combination Therapies: MDSC Modulation Meets Immunotherapy
The most exciting frontier lies in combining MDSC modulation with existing immunotherapies. Checkpoint inhibitors, for instance, unleash T cells but falter against MDSC barricades. Depleting or reprogramming MDSCs prior to ICI administration has shown synergistic effects in melanoma, colorectal, and lung cancer models.
Similarly, cancer vaccines depend on effective antigen presentation and T cell priming—processes actively sabotaged by MDSCs. Reducing MDSCs in the periphery enhances vaccine-induced immunity. Adoptive T cell therapies, including CAR-T cells, also benefit from MDSC modulation, as the infused lymphocytes face fewer suppressive hurdles.
Clinically, early-phase trials combining ATRA with ICIs or CXCR2 antagonists with anti-PD-1 therapy are underway. The outcomes will determine whether MDSC modulation becomes a routine partner to immunotherapy or remains a niche intervention. The guiding principle is clear: attacking the tumor without disarming its cellular allies is a partial victory at best.
Natural Products and Traditional Chinese Medicine
An intriguing dimension of MDSC research is the role of natural compounds and traditional medicine. Several plant-derived agents exhibit immunomodulatory activity capable of reducing MDSC accumulation or function.
For instance, curcumin from turmeric inhibits STAT3 signaling, limiting MDSC expansion. Resveratrol, abundant in grapes, reduces reactive oxygen species production by MDSCs. Ginsenosides, derived from ginseng, modulate differentiation pathways, promoting a shift away from suppressive phenotypes.
Traditional Chinese Medicine (TCM) formulations have attracted attention as multi-component therapies capable of orchestrating complex immunological changes. Herbal combinations have been shown in preclinical studies to reduce MDSC numbers, enhance dendritic cell maturation, and potentiate T cell activity. While mechanistic clarity remains elusive due to the complexity of herbal mixtures, these findings open new avenues for integrative oncology, especially in populations with long-standing traditions of TCM use.
Skeptics may dismiss these approaches as anecdotal, but modern pharmacology increasingly validates what traditional healers intuited: multi-target diseases often require multi-target therapies. MDSC modulation through natural products may complement pharmacological agents, especially when toxicity or resistance limits conventional options.
Challenges and Unanswered Questions
Despite progress, significant challenges remain. Heterogeneity is perhaps the most formidable. MDSCs are not a single entity but a spectrum, with phenotypes varying across cancer types, disease stages, and even between patients. A drug that depletes M-MDSCs in one tumor may leave PMN-MDSCs untouched in another.
Biomarker development is another unmet need. Without reliable ways to quantify MDSCs in blood or tissue, stratifying patients and monitoring therapy becomes guesswork. Flow cytometry panels exist but lack standardization across laboratories. Molecular signatures may help, but clinical translation is slow.
Finally, safety must be considered. MDSCs, for all their sins in cancer, play roles in tissue repair and infection control. Overzealous depletion risks unintended consequences, particularly in patients already immunocompromised by chemotherapy. The art of therapy will be finding the sweet spot: dismantling the tumor’s defenses without dismantling the host’s.
Conclusion
The tale of MDSCs in cancer therapy is one of evolution—from overlooked bystanders to recognized saboteurs, and now to therapeutic targets in their own right. By shaping the immune landscape, MDSCs dictate whether tumors are vulnerable or invincible. Their modulation is not an optional add-on but a prerequisite for the full promise of immunotherapy to be realized.
As oncology moves forward, MDSC-targeted strategies will likely join the expanding list of combination regimens. Whether through pharmacological inhibitors, chemokine antagonists, differentiation agents, or natural products, the principle is the same: neutralize the suppressors to unleash the fighters.
The future of cancer therapy lies not only in attacking tumors but in disarming their allies. MDSCs, once obscure, now sit at the crossroads of this strategy. For patients and clinicians alike, the message is one of cautious optimism: the battlefield is crowded, but victory may come from silencing the tumor’s most faithful soldiers.
FAQ
- Why are MDSCs important in cancer therapy?
MDSCs suppress immune responses in the tumor microenvironment, allowing cancer cells to evade destruction. High levels of MDSCs often predict poor responses to immunotherapy and worse prognosis. - Can MDSCs be completely eliminated?
Probably not without risk. MDSCs also play roles in normal immune regulation. The goal is to reduce or reprogram them enough to restore anti-tumor immunity, not eradicate them entirely. - Are natural products really effective against MDSCs?
Early studies suggest compounds like curcumin, resveratrol, and ginsenosides can reduce MDSC numbers or function. While promising, more rigorous clinical trials are needed before they can be recommended routinely.