Introduction
Prostate cancer remains one of the most prevalent malignancies among men, particularly in the aging population. Advances in screening, diagnosis, and treatment have led to steadily improving survival rates. Yet, as survival improves, a paradox emerges: long-term therapies designed to control tumor growth may themselves introduce metabolic and cardiovascular risks that threaten quality of life and overall health.
Androgen-deprivation therapy (ADT), typically achieved through gonadotropin-releasing hormone (GnRH) agonists such as leuprolide, stands as the cornerstone of systemic treatment for advanced or metastatic prostate cancer. This strategy effectively induces “chemical castration” by reducing testosterone production, thereby suppressing tumor proliferation. However, the benefits of ADT are tempered by well-documented adverse events including sexual dysfunction, fatigue, metabolic syndrome, and an increased risk of cardiovascular disease.
Against this backdrop, sildenafil, a phosphodiesterase type 5 (PDE5) inhibitor, emerges as an intriguing candidate for cardioprotection. Widely known for its role in erectile dysfunction, sildenafil has also demonstrated protective effects against chemotherapy-induced cardiotoxicity and ischemic injury. The natural question arises: could sildenafil mitigate the cardiovascular risks associated with chronic ADT?
A recent preclinical study sought to answer this very question, employing a murine model of long-term leuprolide therapy with or without sildenafil co-administration. The findings were thought-provoking, highlighting both expected and unexpected outcomes.
Establishing the Model: Chemical Castration in Mice
To simulate the clinical setting, researchers administered leuprolide subcutaneously in middle-aged male C57BL/6J mice for 12 weeks. This resulted in marked reductions in serum testosterone levels and prostate weight, confirming effective induction of chemical castration. Importantly, sildenafil did not interfere with the suppression of androgen signaling, alleviating concerns that cardioprotective adjuncts might compromise oncologic efficacy.
The model therefore successfully recapitulated the clinical scenario of men undergoing chronic ADT, providing a reliable foundation for exploring metabolic and cardiovascular consequences.
Metabolic Outcomes: The Hidden Price of Survival
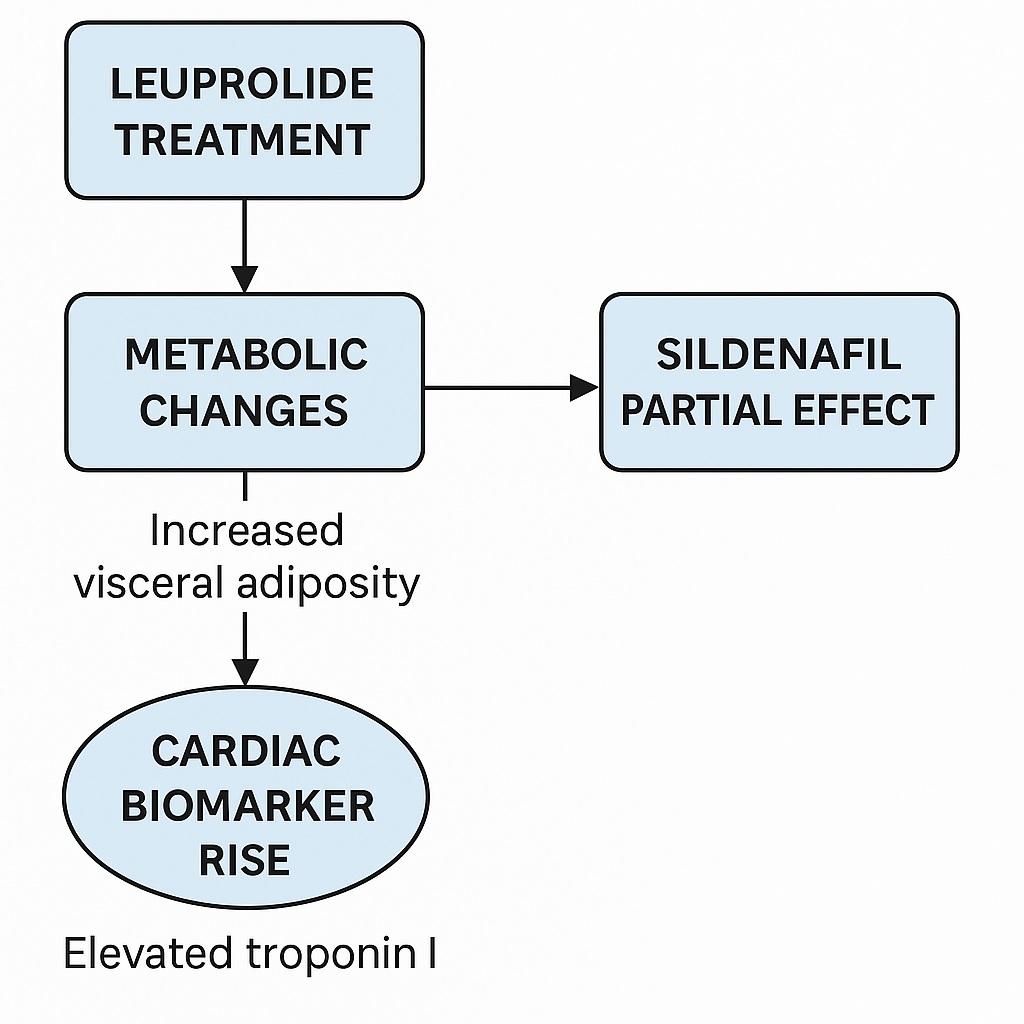
One of the most consistent observations across both clinical and preclinical ADT studies is the tendency toward increased fat accumulation. The mouse model mirrored this phenomenon. After 12 weeks of leuprolide therapy, mice exhibited significantly increased abdominal fat weight despite stable overall body weight.
This distinction is not trivial. The selective expansion of visceral adiposity—rather than generalized weight gain—carries profound implications. Visceral fat is metabolically active, contributing to systemic inflammation, insulin resistance, and dyslipidemia. Collectively, these changes define the metabolic syndrome, which in turn accelerates atherosclerosis and heightens cardiovascular risk.
Interestingly, sildenafil failed to attenuate this pro-adipogenic effect. The drug neither prevented nor reversed the accumulation of abdominal fat, suggesting that PDE5 inhibition does not directly counteract the metabolic derangements induced by ADT. This finding underscores the complexity of ADT-induced metabolic changes, which may involve multifactorial pathways beyond those influenced by cGMP signaling.
Cardiac Function: A Surprising Stability
Given the established cardiovascular concerns surrounding ADT, researchers anticipated potential declines in cardiac function. Echocardiography was performed at baseline and throughout the 12-week treatment period, assessing left ventricular ejection fraction (LVEF), heart rate, and diastolic function.
Contrary to expectations, no overt systolic or diastolic dysfunction was observed in leuprolide-treated mice. LVEF, heart rate, and E/A ratios remained comparable to controls, even after three months of continuous therapy. This apparent resilience of cardiac mechanics raises several possibilities.
First, it is conceivable that the murine heart possesses adaptive mechanisms buffering against the metabolic stress of ADT in the short term. Second, the relatively healthy status of middle-aged mice may not fully capture the vulnerability of elderly human patients with preexisting comorbidities such as diabetes, hypertension, or prior myocardial infarction. Third, the 12-week duration, while substantial for mice, may not entirely equate to the multi-year exposure typical in clinical practice.
Thus, while the absence of frank cardiac dysfunction is reassuring, it should be interpreted with caution when extrapolating to human populations.
Cardiac Biomarkers: A Signal in the Noise
Perhaps the most intriguing finding was the elevation of serum cardiac troponin I (cTn-I) following leuprolide treatment. Troponin I is a highly sensitive biomarker of cardiomyocyte injury and is widely used clinically to diagnose myocardial infarction.
Despite stable echocardiographic parameters, leuprolide-treated mice demonstrated a significant rise in cTn-I levels, suggestive of subclinical myocardial injury. This dissociation between structural function and biochemical markers mirrors observations in patients receiving anthracycline chemotherapy, where troponin elevations may precede detectable declines in LVEF.
The implication is sobering: ADT may inflict subtle myocardial damage that remains invisible on imaging but could accumulate over time to manifest as overt cardiac dysfunction. Monitoring of troponin levels in clinical practice might therefore serve as an early warning system for ADT-related cardiotoxicity.
Sildenafil: A Cardiac Guardian That Fell Short?
Given sildenafil’s well-documented cardioprotective effects in other contexts, investigators hypothesized that co-treatment might blunt leuprolide’s adverse outcomes. The reality proved more complex.
While sildenafil did not disrupt chemical castration or prostate regression, it also failed to prevent abdominal adiposity or cTn-I elevation. Some variability was noted, with subsets of mice showing partial mitigation of troponin rise, but the overall effect was inconsistent.
This lack of uniform benefit raises questions. Were the doses optimal? Did sildenafil require longer exposure or specific timing to exert cardioprotection? Could genetic variability among mice account for the divergent responses? These uncertainties highlight the need for further mechanistic exploration before sildenafil can be confidently positioned as a cardioprotective adjunct to ADT.
Translating Findings to Human Context
The murine model offers valuable insights, but clinical translation demands careful nuance. Human patients receiving ADT are typically older, frequently harboring comorbidities that amplify cardiovascular risk. Epidemiological studies indeed show higher incidences of diabetes, coronary artery disease, and sudden cardiac death among ADT recipients, though results remain somewhat inconsistent.
The elevation of cTn-I observed in mice aligns with recent human studies where troponin rises were documented after six months of ADT, even in the absence of overt heart failure. Such concordance lends credibility to the murine findings and suggests that subclinical myocardial injury may represent a genuine concern in clinical practice.
However, sildenafil’s disappointing performance in mice tempers enthusiasm for its immediate adoption as a protective strategy. Future trials must clarify whether PDE5 inhibitors can meaningfully mitigate ADT cardiotoxicity in human populations, perhaps in selected patient subsets or when combined with other interventions.
Clinical Implications: A Balancing Act
The take-home message is not to abandon ADT—it remains a life-prolonging therapy without which prostate cancer outcomes would be markedly worse. Rather, clinicians must balance oncologic efficacy against long-term systemic risks. Several strategies merit consideration:
- Careful baseline cardiovascular assessment before initiating ADT.
- Regular monitoring of metabolic parameters, including glucose, lipids, and body composition.
- Incorporation of troponin testing as a potential biomarker of early myocardial injury.
- Lifestyle interventions targeting diet, exercise, and weight management to counteract visceral adiposity.
- Selective use of adjunctive agents, such as statins or antihypertensives, tailored to individual risk profiles.
The role of sildenafil and other PDE5 inhibitors remains unresolved. While they may not universally shield the heart from ADT-induced injury, ongoing research may yet reveal subgroups or conditions where benefit emerges.
Future Directions
This study raises more questions than it answers—a hallmark of meaningful scientific work. Key avenues for further exploration include:
- Mechanistic dissection of how leuprolide drives adipogenesis and myocardial injury at the molecular level.
- Longitudinal studies extending beyond 12 weeks to assess delayed manifestations of cardiac dysfunction.
- Evaluation of sildenafil and other PDE5 inhibitors at varying doses, durations, and in combination with additional cardioprotective agents.
- Clinical trials incorporating troponin monitoring as an endpoint, enabling early intervention before irreversible cardiac damage occurs.
Ultimately, the goal is to refine ADT protocols that maximize cancer control while minimizing collateral damage to metabolism and cardiovascular health.
Conclusion
Androgen-deprivation therapy with leuprolide remains indispensable in the management of advanced prostate cancer. Yet, as this preclinical study demonstrates, the therapy is not without cost. Increased visceral adiposity and biochemical evidence of cardiac injury, even in the absence of functional decline, underscore the importance of vigilance.
Sildenafil, while promising in other settings, failed to deliver consistent cardioprotection in this model. Its role in clinical practice therefore remains uncertain, pending further investigation.
For clinicians, the message is clear: treat prostate cancer aggressively, but not blindly. Monitor, anticipate, and mitigate the unintended consequences of life-prolonging therapies. For researchers, the work continues—deciphering mechanisms, testing interventions, and striving for a future where survival and quality of life are no longer in conflict.
FAQ
1. Does androgen-deprivation therapy always cause heart problems?
Not always. While many patients tolerate ADT without overt cardiac dysfunction, studies suggest an increased risk of cardiovascular events, particularly in those with preexisting risk factors. Monitoring and preventive strategies can reduce this risk.
2. Can sildenafil protect the heart during ADT?
Current evidence, including this preclinical study, does not support a consistent cardioprotective effect of sildenafil in the context of ADT. More research is needed before it can be recommended for this purpose.
3. Should troponin levels be monitored in patients on ADT?
Emerging data suggest that troponin may rise before detectable cardiac dysfunction occurs, serving as an early warning signal. While not yet standard practice, troponin monitoring may become an important tool in managing long-term ADT patients.