Functional hypogonadism is increasingly recognized as one of the silent medical epidemics of the 21st century. Unlike classical hypogonadism caused by irreversible testicular or pituitary failure, functional hypogonadism reflects a state of reversible testosterone deficiency often driven by lifestyle and metabolic factors. It emerges at the crossroads of obesity, sedentary behavior, metabolic syndrome, and aging. Its relevance extends beyond endocrinology, as it influences urology, cardiology, psychiatry, andrology, and reproductive medicine.
The purpose of this article is to unravel the mechanisms underpinning functional hypogonadism, highlight the clinical challenges, and review evidence for lifestyle and non-pharmacological interventions, while not ignoring the occasional necessity of pharmacological aids. The message is clear: while testosterone replacement therapy (TRT) has its place, a growing body of evidence demonstrates that sustainable reversal of functional hypogonadism is best achieved by addressing its root causes.
Defining Functional Hypogonadism
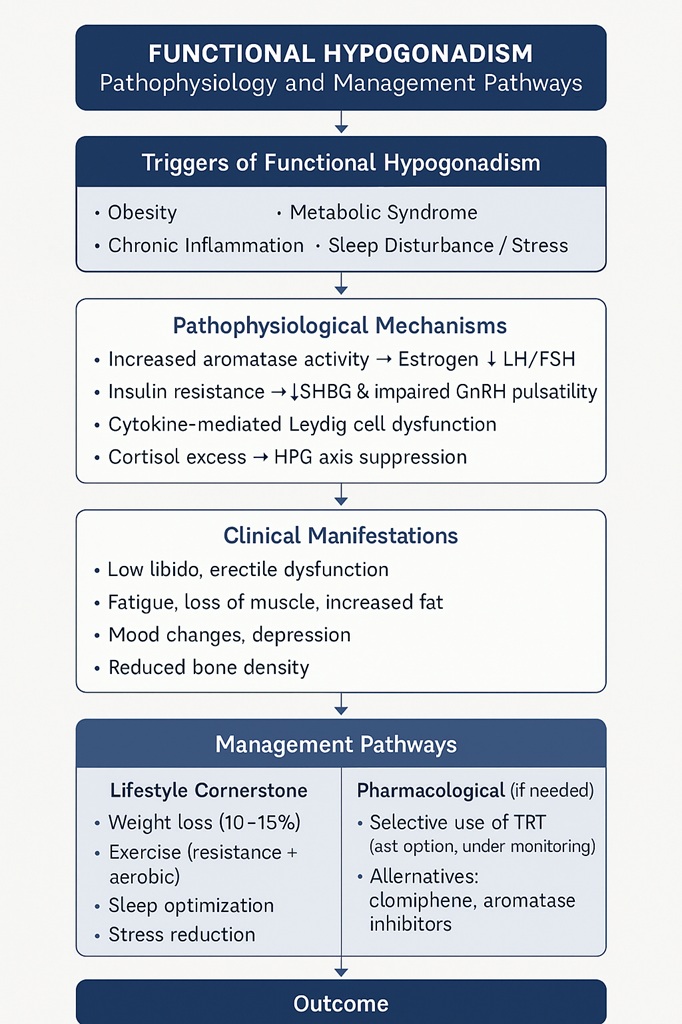
Functional hypogonadism refers to a clinical condition where circulating testosterone levels fall below normal, accompanied by characteristic symptoms such as reduced libido, erectile dysfunction, decreased muscle mass, increased fat accumulation, and impaired mood. Unlike organic hypogonadism, there is no structural lesion in the testes or hypothalamic–pituitary axis. Instead, this condition is triggered by reversible influences, primarily obesity, metabolic syndrome, chronic stress, systemic inflammation, and sleep disturbances.
This concept challenges traditional endocrinology. For decades, physicians were trained to think of hypogonadism as a permanent endocrine failure requiring lifelong replacement. Yet, functional hypogonadism is best understood as an adaptive suppression of the hypothalamic–pituitary–gonadal (HPG) axis. In obese or metabolically unhealthy men, excessive adiposity drives aromatase activity, increasing estrogen production and feeding back negatively on gonadotropin secretion. Pro-inflammatory cytokines add further suppression, creating a downward spiral.
Importantly, functional hypogonadism is not confined to elderly men. Young and middle-aged men increasingly present with the condition, correlating with rising global obesity. Recognizing the difference between functional and organic hypogonadism is therefore critical—not just for treatment, but for prognosis. While the latter may demand lifelong TRT, the former can often be reversed through targeted interventions.
Pathophysiology: A Metabolic–Endocrine Crossroad
The suppression of testosterone in functional hypogonadism is multifactorial, reflecting the intersection of endocrine and metabolic dysfunction. Several key mechanisms contribute:
- Obesity and Aromatase Activity: Excess adipose tissue converts testosterone into estradiol via aromatase, amplifying estrogen-mediated feedback inhibition on gonadotropin secretion.
- Inflammation: Cytokines such as TNF-α and IL-6 directly impair Leydig cell steroidogenesis.
- Insulin Resistance: Hyperinsulinemia suppresses sex hormone-binding globulin (SHBG) and interferes with GnRH pulsatility.
- Sleep and Stress: Poor sleep and chronic cortisol elevation blunt nocturnal testosterone surges.
The net result is low serum testosterone, frequently accompanied by normal or low gonadotropins, mimicking central hypogonadism. This endocrine suppression is not a sign of irreversible organ failure but an adaptive mechanism, almost as if the body were choosing to conserve reproductive resources until metabolic homeostasis is restored. Unfortunately, the price of this adaptation is compromised quality of life and increased cardiometabolic risk.
Clinical Presentation and Diagnosis
Patients with functional hypogonadism typically present with non-specific symptoms that overlap with other metabolic disorders. Complaints often include fatigue, loss of energy, reduced libido, erectile dysfunction, and decreased morning erections. Sarcopenia and visceral obesity further amplify the clinical picture. Some patients report mood disturbances ranging from irritability to frank depression.
Diagnosing functional hypogonadism requires careful evaluation. Biochemical confirmation involves measuring morning total testosterone on at least two occasions, alongside SHBG and luteinizing hormone (LH) to determine the pattern. In functional cases, testosterone is low but the gonadotropins are inappropriately normal or low, consistent with a central suppression rather than testicular failure.
Importantly, clinicians must exclude primary organic causes such as Klinefelter syndrome, pituitary adenomas, or testicular injury. Once these are ruled out, and especially in the presence of obesity, metabolic syndrome, or type 2 diabetes, functional hypogonadism becomes the likely diagnosis. Too often, physicians reach reflexively for TRT without exploring lifestyle-driven reversibility—a missed opportunity for genuine recovery.
Why Testosterone Matters Beyond Sexual Health
The relevance of testosterone extends far beyond libido and erections. It is a systemic hormone influencing multiple organ systems. In muscle, testosterone stimulates protein synthesis and reduces fat infiltration. In bone, it maintains mineral density. In the brain, it modulates mood, cognition, and motivation. Cardiometabolic health also depends on optimal testosterone: low levels are associated with insulin resistance, dyslipidemia, and endothelial dysfunction.
The bidirectional relationship between testosterone and metabolic health is striking. On one hand, metabolic syndrome suppresses testosterone. On the other, hypogonadism exacerbates insulin resistance, adiposity, and inflammation, perpetuating a vicious cycle. Breaking this loop is the therapeutic priority. It is not just about restoring sexual vitality; it is about rescuing long-term health outcomes. Men with untreated functional hypogonadism face increased risks of cardiovascular events and reduced life expectancy.
Lifestyle Modification: The Cornerstone of Treatment
The cornerstone of reversing functional hypogonadism is lifestyle intervention. Unlike organic hypogonadism, where hormone replacement is the only option, functional hypogonadism responds impressively to weight loss, exercise, dietary changes, and sleep optimization.
Weight Loss as Hormonal Therapy
Multiple studies demonstrate that weight loss of 10–15% can normalize testosterone in obese men. Bariatric surgery provides dramatic improvements, but even structured diet programs lead to measurable increases. The reduction in visceral fat decreases aromatase activity, lowers inflammation, and restores hypothalamic sensitivity to gonadotropin-releasing hormone (GnRH). In many cases, testosterone levels rise by 200–300 ng/dL without pharmacological intervention.
Exercise and Resistance Training
Exercise not only burns calories but also directly stimulates testosterone release. Resistance training is particularly effective, with compound lifts like squats and deadlifts known to transiently raise testosterone and growth hormone. More importantly, regular training increases lean body mass, reduces insulin resistance, and enhances SHBG regulation. Long-term adherence reshapes the hormonal milieu. Aerobic activity complements this by reducing visceral fat and improving cardiovascular resilience.
Sleep and Stress Management
Chronic sleep restriction reduces testosterone by up to 15% within a week, underscoring the importance of restorative sleep. Addressing sleep apnea, a common comorbidity in obese men, can normalize testosterone without further intervention. Stress reduction strategies, from mindfulness to cognitive behavioral therapy, lower cortisol and improve hypothalamic signaling.
Taken together, lifestyle modification acts as the most physiological form of testosterone therapy. Unlike TRT, which risks shutting down endogenous production, lifestyle changes revive the body’s own capacity for steroidogenesis.
Non-Pharmacological Adjuncts
While lifestyle forms the foundation, additional non-pharmacological strategies support recovery. Nutritional optimization plays a key role. Adequate protein intake ensures substrate availability for muscle synthesis. Micronutrients such as vitamin D, zinc, and magnesium support steroidogenesis. Omega-3 fatty acids modulate inflammation, indirectly aiding the HPG axis.
Psychological counseling may also be essential. Many men with functional hypogonadism struggle with low motivation and poor adherence to health plans. Structured behavioral interventions increase compliance with exercise and diet, ensuring sustainability. Sexual counseling can reduce performance anxiety, a frequent accomplice to organic dysfunction.
Interestingly, certain non-testosterone pharmacological agents—such as PDE5 inhibitors like sildenafil—sometimes enter the equation. While not curative, they provide symptomatic relief of erectile dysfunction, buying time while lifestyle interventions take effect. Their role underscores a principle of pragmatic medicine: sometimes patients need short-term tools while working toward long-term recovery.
Testosterone Therapy: Use with Caution
There remains a controversial place for testosterone therapy in functional hypogonadism. Guidelines caution against indiscriminate prescription, yet the practice persists. The temptation is understandable: TRT provides rapid symptomatic improvement, boosting energy and libido within weeks. However, in functional cases, this may mask the underlying problem while risking suppression of endogenous production.
The ideal candidates for TRT are those who fail to respond to sustained lifestyle interventions, or those with severe symptomatic hypogonadism compromising quality of life. Even then, physicians should emphasize that TRT is not a substitute for weight management or metabolic control. Regular monitoring of hematocrit, prostate health, and cardiovascular risk is essential.
An alternative approach involves selective therapies that stimulate endogenous testosterone, such as clomiphene citrate or aromatase inhibitors, though these remain less commonly used in routine practice. They highlight the principle that preserving physiological function is preferable to replacing it outright.
Functional Hypogonadism and Public Health
Beyond the individual, functional hypogonadism poses significant public health challenges. It mirrors the global rise of obesity and metabolic syndrome, both of which have reached epidemic proportions. The decline in average male testosterone levels observed in population studies is not simply an artifact of aging demographics but a reflection of societal lifestyle shifts—sedentarism, poor diet, chronic stress, and inadequate sleep.
Addressing functional hypogonadism is therefore not just about prescribing hormones. It requires systemic interventions—public health campaigns promoting physical activity, nutritional literacy, sleep hygiene, and obesity prevention. Focusing solely on TRT would be like prescribing inhalers during a smog crisis without tackling air pollution. True prevention lies in reshaping environments that foster metabolic dysfunction.
Looking Ahead: Research and Innovation
Research on functional hypogonadism continues to expand, with several promising areas. Studies are exploring the genetic determinants of individual susceptibility, explaining why some obese men develop hypogonadism while others do not. Advances in wearable technology allow real-time tracking of sleep, activity, and stress, offering opportunities for personalized interventions.
Novel therapeutics are also being evaluated. Agents targeting inflammation, metabolic pathways, and even gut microbiota may indirectly restore testosterone levels. The role of digital health coaching—combining telemedicine with behavioral tracking—has gained traction in sustaining lifestyle interventions.
Importantly, ongoing trials are clarifying the long-term safety of TRT in functional hypogonadism, weighing symptomatic benefits against potential cardiovascular risks. The future likely lies in a hybrid approach: judicious pharmacological support combined with rigorous lifestyle correction.
Conclusion
Functional hypogonadism is a reversible form of testosterone deficiency born from modern lifestyles. Unlike organic hypogonadism, it does not signify endocrine failure but rather metabolic suppression of reproductive capacity. Its consequences, however, are far from benign, affecting sexual health, physical strength, mood, and long-term cardiometabolic outcomes.
The evidence is compelling: weight loss, exercise, improved sleep, and stress reduction can restore testosterone levels and quality of life. Pharmacological therapies, including TRT, should be reserved for carefully selected patients and ideally combined with lifestyle measures. Ultimately, treating functional hypogonadism is not about chasing laboratory numbers but about restoring vitality, health, and resilience.
The physician’s task is both clinical and motivational—guiding patients toward choices that empower their physiology rather than replacing it. In many ways, functional hypogonadism teaches us a timeless medical truth: the most powerful medicines are not always dispensed from a vial but forged in the rhythms of daily life.
FAQ
1. Can functional hypogonadism be permanently cured without testosterone therapy?
Yes. In most cases, functional hypogonadism is reversible through sustained lifestyle interventions such as weight loss, regular exercise, and improved sleep. Unlike organic hypogonadism, it does not require lifelong TRT unless the underlying metabolic drivers remain unaddressed.
2. How long does it take for testosterone to improve after lifestyle changes?
Significant improvements can occur within 3–6 months of consistent weight loss and exercise, though full recovery may take longer. Patience and persistence are key, as metabolic adaptation is gradual.
3. Is testosterone replacement therapy dangerous for men with functional hypogonadism?
TRT is not inherently dangerous but must be used cautiously. It may improve symptoms quickly but risks suppressing natural testosterone production and carries potential cardiovascular and hematological risks. It should be considered only when lifestyle modification fails and under close medical supervision.