Introduction
Infertility remains one of the most emotionally taxing conditions in reproductive medicine, and the role of endometrial receptivity is central to both natural conception and assisted reproductive technologies (ART). Among the many determinants of endometrial receptivity, endometrial thickness occupies a privileged position, not because it is the only parameter that matters, but because it is one of the few that can be easily visualized, quantified, and, in theory, manipulated. A persistently thin endometrium—often defined as less than 7 mm at the time of ovulation induction or embryo transfer—has long been associated with lower implantation rates, diminished pregnancy outcomes, and frustrating cycles for both clinicians and patients.
For decades, the pharmacological toolkit available to address thin endometrium was both limited and largely disappointing. Estrogen supplementation, the cornerstone intervention, is far from universally effective. Other approaches—ranging from low-dose aspirin to growth factors and stem cell therapies—have shown promise but remain variably accessible and inconsistently effective. Against this background, sildenafil citrate, a drug that entered the collective consciousness as the archetypal therapy for erectile dysfunction, has taken on an unexpected new role in gynecology. The concept is deceptively simple: if sildenafil can improve penile vascular perfusion through selective inhibition of phosphodiesterase-5 (PDE-5), perhaps it can also enhance endometrial blood flow, stimulate growth, and improve receptivity.
The transition from urology to reproductive medicine may seem unconventional, but scientific curiosity has always thrived on boundary crossing. In this article, we will explore in detail the role of sildenafil in women with thin endometrium, focusing strictly on its clinical, pharmacological, and therapeutic relevance in infertility management.
Pathophysiology of Thin Endometrium
Thin endometrium is not a diagnosis in itself but a clinical manifestation of multiple underlying disturbances. Its etiology can range from chronic endometritis and intrauterine adhesions to long-term clomiphene use, repeated curettage, or idiopathic factors that resist clear explanation. Regardless of cause, the consequence is a failure of the endometrium to undergo the proliferative changes necessary to support implantation.
Histologically, a thin endometrium often demonstrates reduced glandular density, poor vascularization, and suboptimal stromal development. The molecular signature is equally concerning: downregulation of angiogenic factors, diminished nitric oxide (NO) signaling, and inadequate expression of implantation-related genes. These deficiencies culminate in an environment where even high-quality embryos struggle to implant successfully.
What makes thin endometrium particularly challenging is its resilience against conventional therapies. While exogenous estrogen should, in principle, stimulate proliferation, in many patients the expected response never materializes. This resistance has spurred the investigation of adjunctive treatments, among which sildenafil has emerged as a particularly intriguing candidate.
Pharmacological Rationale for Sildenafil Use
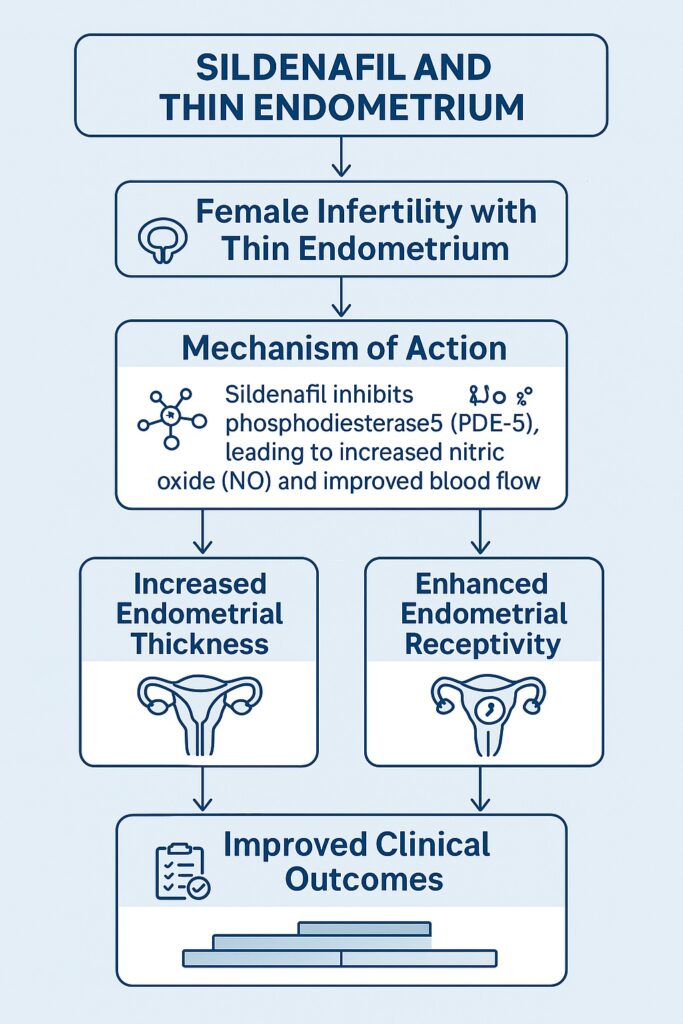
Sildenafil is a selective PDE-5 inhibitor, originally developed to treat angina pectoris but serendipitously repurposed for erectile dysfunction. Its primary mechanism involves the inhibition of PDE-5, an enzyme responsible for degrading cyclic guanosine monophosphate (cGMP). By preventing cGMP breakdown, sildenafil prolongs smooth muscle relaxation mediated by nitric oxide, thereby enhancing blood flow to vascular tissues.
In the endometrium, where angiogenesis and blood flow are critical for growth and receptivity, sildenafil’s pharmacological effects can, in theory, overcome one of the core limitations of thin endometrium: insufficient perfusion. Animal studies have supported this hypothesis, demonstrating improved uterine artery blood flow after sildenafil administration. Moreover, increased vascular endothelial growth factor (VEGF) expression has been observed, suggesting an effect that extends beyond hemodynamics to molecular drivers of angiogenesis.
One of the ironies in reproductive pharmacology is that a drug designed for male sexual dysfunction has gained traction as a potential remedy for female infertility. But such ironies are not rare in medicine—after all, aspirin was never intended as a cardioprotective agent, and yet it remains a cornerstone of cardiovascular prevention. Sildenafil’s journey into the fertility clinic may be unorthodox, but it is not without precedent.
Clinical Evidence: Sildenafil in Thin Endometrium
The most compelling evidence for sildenafil’s role comes from small randomized controlled trials, observational studies, and more recently, systematic reviews and meta-analyses. The collective data converge on a few key findings:
First, sildenafil administration—whether oral or vaginal—has been shown to increase endometrial thickness in women who previously failed to respond adequately to estrogen therapy. In some studies, endometrial thickness improved from an average of less than 6 mm to over 8 mm, a change that can mean the difference between cycle cancellation and embryo transfer.
Second, clinical outcomes reflect these morphological improvements. Women treated with sildenafil demonstrated higher implantation rates, improved clinical pregnancy rates, and, in some cohorts, better live birth outcomes compared with controls. Although the absolute differences vary across studies, the consistency of direction lends credibility to the effect.
Third, the safety profile appears favorable. Reported adverse effects are generally mild—headaches, flushing, or transient dizziness—and tend to resolve spontaneously. Importantly, no significant maternal or fetal complications have been documented in the limited populations studied, though long-term safety data remain incomplete.
These findings have collectively elevated sildenafil from a speculative intervention to a legitimate therapeutic candidate for women with thin endometrium, particularly those undergoing ART.
Routes of Administration: Oral vs. Vaginal Sildenafil
An important practical consideration is how sildenafil is administered. Oral sildenafil, at doses comparable to those used in erectile dysfunction, is the most straightforward approach. However, first-pass hepatic metabolism reduces systemic bioavailability, and concerns exist about whether sufficient drug concentration reaches the uterus.
Vaginal administration offers a theoretically superior alternative. By bypassing first-pass metabolism, vaginal sildenafil can deliver higher local concentrations to the pelvic organs with lower systemic exposure. Clinical studies comparing the two routes suggest that vaginal administration may result in more pronounced improvements in endometrial thickness and uterine blood flow, though direct head-to-head trials remain limited.
From a patient perspective, the choice between oral and vaginal sildenafil is not trivial. Oral administration is less invasive and more socially acceptable, while vaginal suppositories may be less convenient but potentially more effective. Ultimately, the route of administration should be individualized, guided by patient preference, tolerability, and clinical response.
Limitations of the Current Evidence
While the promise of sildenafil is undeniable, the limitations of current evidence should not be overlooked. Most studies are small, single-center, and often include heterogeneous patient populations. Definitions of “thin endometrium” vary, as do outcome measures. Some trials focus on endometrial thickness, while others prioritize pregnancy or live birth rates. The lack of standardization makes meta-analysis challenging and contributes to variability in reported effect sizes.
Moreover, publication bias cannot be dismissed. Studies with positive results are more likely to be published, particularly in a field where novel therapeutic interventions attract considerable attention. Without large-scale, multicenter randomized controlled trials, the true magnitude of sildenafil’s benefit remains uncertain.
Finally, the long-term safety of sildenafil use in women attempting conception is insufficiently studied. While no red flags have been raised thus far, the absence of evidence is not evidence of absence. Ongoing surveillance and cautious optimism should guide clinical use.
Clinical Integration and Practical Considerations
For clinicians, the integration of sildenafil into infertility practice raises both opportunities and questions. On the one hand, sildenafil offers a plausible, relatively low-cost, and accessible intervention for a frustrating clinical problem. On the other, its use remains off-label, with no universally accepted protocols regarding dosage, timing, or route of administration.
Some reproductive centers have already adopted sildenafil as part of their armamentarium for patients with refractory thin endometrium, often in conjunction with high-dose estrogen therapy. Anecdotally, many clinicians report meaningful improvements in cycle outcomes, lending pragmatic support to the evidence base. Yet the absence of regulatory approval creates an environment of cautious experimentation rather than standardized care.
Until more definitive evidence emerges, the most prudent approach is to offer sildenafil selectively, particularly to women who have repeatedly failed to achieve adequate endometrial thickness with conventional therapies. In such cases, the potential benefits likely outweigh the modest risks, especially when patients are counseled transparently about the off-label nature of treatment.
Future Directions
The trajectory of sildenafil in reproductive medicine is still in its early stages. Future research must prioritize large randomized controlled trials that clearly define patient populations, standardize treatment protocols, and report outcomes that matter most—live birth rates rather than surrogate markers.
Beyond traditional trial design, mechanistic studies are also needed. Understanding precisely how sildenafil influences endometrial angiogenesis, gene expression, and implantation biology will not only validate its current use but may also inspire novel therapeutic targets.
Moreover, combination therapies warrant exploration. Sildenafil may be synergistic with other interventions, such as granulocyte colony-stimulating factor or stem cell therapies, offering a multifaceted approach to thin endometrium. As history has shown, progress in reproductive medicine often comes not from a single breakthrough but from the convergence of multiple complementary strategies.
Conclusion
The repurposing of sildenafil from a treatment for erectile dysfunction to a potential solution for thin endometrium illustrates both the creativity and pragmatism of modern reproductive medicine. While the evidence is still evolving, current data suggest that sildenafil can improve endometrial thickness, enhance receptivity, and increase pregnancy rates in women who previously faced dismal prospects.
To be clear, sildenafil is not a panacea. Its efficacy is not universal, its protocols are not standardized, and its long-term safety remains incompletely understood. Yet for women struggling with thin endometrium—a problem that can sabotage even the most advanced ART cycles—sildenafil represents a beacon of hope, and perhaps a new chapter in the pharmacological management of infertility.
The irony of its journey from the male bedroom to the female fertility clinic is not lost on anyone. But if sildenafil ultimately helps more women achieve successful pregnancies, then medicine has once again demonstrated its most admirable trait: the ability to adapt, repurpose, and innovate in the service of human life.
FAQ
1. Is sildenafil officially approved for treating thin endometrium in women?
No. Sildenafil use in this context is off-label. While evidence suggests benefits, regulatory bodies have not yet approved it for infertility management.
2. Which route of administration is more effective: oral or vaginal?
Both have shown benefits, but vaginal administration may achieve higher local drug concentrations with fewer systemic effects. Direct comparative trials are still limited.
3. Is sildenafil safe for women trying to conceive?
Current data suggest that sildenafil is generally well-tolerated, with mild side effects like headache and flushing. However, long-term maternal and fetal safety data remain insufficient, so careful clinical judgment is necessary.