Introduction
Lower urinary tract symptoms (LUTSs) and erectile dysfunction (ED) represent two of the most common and bothersome conditions affecting middle-aged and elderly men. For decades, they were treated as independent clinical entities: LUTSs as the inevitable outcome of benign prostatic hyperplasia (BPH), and ED as a stand-alone sexual disorder tied to aging, vascular disease, or hormonal imbalance. Yet, accumulating epidemiological evidence has forced clinicians to acknowledge an inconvenient truth: LUTSs and ED are not merely coexisting, they are tightly interwoven conditions.
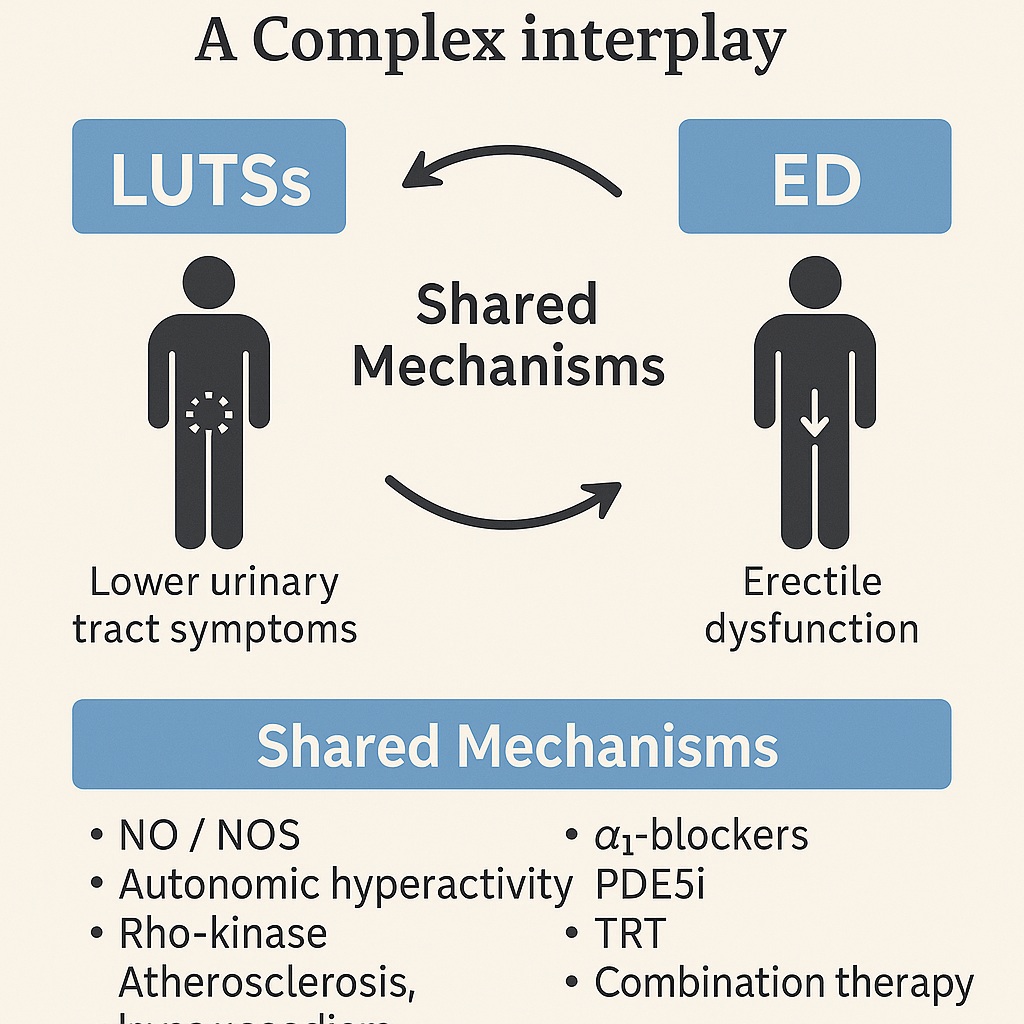
Men who suffer from LUTSs are significantly more likely to develop ED, and the severity of urinary symptoms often mirrors the severity of erectile impairment. Importantly, this association persists even after controlling for age, lifestyle, and comorbidities such as diabetes or cardiovascular disease. The overlap is not coincidental; instead, it reflects shared pathophysiological pathways that influence both bladder–prostate function and penile erection.
This review explores the clinical, mechanistic, and therapeutic intersections between LUTSs and ED, highlighting how an integrated approach can enhance both urological and sexual health in aging men.
Epidemiological Evidence: Numbers Do Not Lie
The link between LUTSs and ED is not speculative; it is grounded in large-scale population studies across continents. Cross-sectional analyses consistently reveal that men with moderate to severe LUTSs are two to seven times more likely to experience ED than asymptomatic peers. The Multinational Survey of the Aging Male (MSAM-7), which included more than 12,000 men from diverse backgrounds, demonstrated a dose–response relationship: the worse the urinary symptoms, the higher the probability and severity of erectile dysfunction.
Interestingly, both storage symptoms (urgency, frequency, nocturia) and voiding symptoms (hesitancy, weak stream, incomplete emptying) contribute to ED risk, though studies differ on which type carries greater weight. Some European cohorts emphasized obstructive symptoms, whereas Asian studies highlighted irritative features such as nocturia and urgency. Regardless of regional variation, the correlation remains strikingly consistent.
Longitudinal studies, though fewer in number, strengthen the case for causality. In Finnish and Brazilian cohorts, baseline LUTSs predicted subsequent development of ED over several years, with risk rising proportionally with symptom severity. Conversely, evidence for ED leading to LUTSs is weaker, suggesting a directional influence from urinary dysfunction toward sexual dysfunction.
The conclusion is clear: LUTSs are an independent risk factor for ED, not just a coincidental companion of aging.
Pathophysiological Bridges between LUTSs and ED
Why do problems with the bladder and prostate so often coexist with erectile impairment? Four main mechanistic theories have emerged, each offering pieces of the puzzle.
The Nitric Oxide/NOS Theory
Erection depends on nitric oxide (NO)-mediated relaxation of penile smooth muscle through cyclic guanosine monophosphate (cGMP). A similar mechanism regulates smooth muscle tone in the bladder neck and prostate. Reduced NO synthase expression in BPH tissues, diminished nitrergic innervation, and impaired urothelial NO release all contribute to both urinary obstruction and erectile dysfunction. Simply put, a deficiency in the same molecular pathway derails two systems simultaneously.
Autonomic Hyperactivity
Men with LUTSs frequently exhibit heightened sympathetic tone, a hallmark of metabolic syndrome. Chronic sympathetic overdrive not only fuels prostate growth and bladder overactivity but also inhibits penile erection. Animal models confirm that manipulation of autonomic activity alters both prostate physiology and erectile function, underscoring the systemic nature of this dysfunction.
The Rho-Kinase Pathway
Rho-kinase signaling amplifies smooth muscle contraction in the corpus cavernosum and lower urinary tract. Abnormal upregulation of this pathway has been observed in bladder outlet obstruction models, where it increases contractility, reduces relaxation, and disrupts neuromuscular signaling. In effect, excessive Rho-kinase activity locks smooth muscles in a contracted state, impairing both urination and erection.
Pelvic Atherosclerosis
A more structural explanation points to vascular insufficiency. Diffuse atherosclerosis compromises blood flow to the bladder, prostate, and penis, resulting in ischemia, fibrosis, and loss of compliance. This vascular theory neatly integrates LUTSs and ED into the broader spectrum of endothelial dysfunction and cardiovascular disease, positioning them as early markers of systemic vascular compromise.
Together, these mechanisms highlight that LUTSs and ED are not separate misfortunes but parallel expressions of shared pathophysiology.
The Testosterone Factor
While vascular and neural mechanisms dominate the discussion, hormones—particularly testosterone—deserve attention. Approximately 20% of men with ED have low serum testosterone, and hypogonadism often coexists with LUTSs. Low testosterone is associated with increased metabolic and vascular burden, diminished NO synthesis, and compromised smooth muscle integrity in the penis and bladder.
Experimental data suggest that androgens help preserve cavernosal architecture and promote bladder neck relaxation. Clinical trials, albeit small, indicate that testosterone replacement therapy (TRT) improves both erectile function and LUTSs in hypogonadal men, particularly when combined with PDE5 inhibitors. These findings elevate testosterone from a peripheral player to a central factor in the LUTS–ED connection.
Therapeutic Intersections
The silver lining in this complex relationship is therapeutic synergy: treatments developed for one condition often benefit the other.
α1-Blockers for ED
Traditionally prescribed for LUTSs due to BPH, α1-blockers (e.g., alfuzosin, doxazosin) relax smooth muscle in the prostate and bladder neck. Interestingly, they also facilitate penile erection by counteracting adrenergic-mediated detumescence. Clinical studies confirm that men with BPH/LUTSs who receive α1-blockers often report improved erectile function as a welcome side effect.
PDE5 Inhibitors for LUTSs
Conversely, PDE5 inhibitors (sildenafil, tadalafil, vardenafil) primarily developed for ED, demonstrate significant benefits for LUTSs. By enhancing NO–cGMP signaling in the bladder, urethra, and prostate, they reduce symptom scores even without increasing urinary flow rates. Their effect is particularly notable on storage symptoms, suggesting an influence on detrusor overactivity and bladder compliance.
Combination Therapy
For men burdened with both LUTSs and ED, combination therapy appears logical. α1-blockers and PDE5 inhibitors act through distinct but complementary mechanisms, offering dual relief. Pilot trials suggest superior outcomes with combination therapy compared to monotherapy. However, clinicians must exercise caution due to risks of hypotension when both drug classes are combined, particularly in older men or those on antihypertensives.
Testosterone Replacement
In hypogonadal men, TRT alone or in combination with PDE5 inhibitors has shown improvements in both urinary and sexual domains. Small randomized studies reveal increased bladder capacity, reduced detrusor pressure, improved flow rates, and better erectile quality. Though promising, these results require validation in larger trials before TRT can be universally endorsed as a LUTS–ED therapy.
Clinical Implications
The coexistence of LUTSs and ED is more than an inconvenience; it is a diagnostic and therapeutic opportunity. Recognizing ED in a patient with LUTSs should prompt screening for cardiovascular and metabolic disease, given the shared pathophysiology. Conversely, men presenting with ED may harbor unreported urinary symptoms requiring attention.
An integrated management strategy should therefore include:
- Routine inquiry about both urinary and sexual function in men over 50.
- Risk factor modification targeting metabolic syndrome, hypertension, and vascular disease.
- Personalized pharmacological regimens that maximize dual benefits (e.g., PDE5 inhibitors for both conditions).
- Consideration of hormonal evaluation and TRT in symptomatic hypogonadal men.
Such a holistic approach not only improves symptom control but also enhances overall quality of life, addressing the dual burdens of LUTSs and ED in one coordinated strategy.
Conclusion
The days of treating LUTSs and ED as isolated disorders are over. Epidemiological evidence, mechanistic insights, and therapeutic overlaps converge on a single reality: LUTSs and ED are intimately linked manifestations of aging male health. Their association is underpinned by NO deficiency, autonomic dysregulation, Rho-kinase activity, pelvic atherosclerosis, and hypogonadism.
Fortunately, treatments are increasingly versatile. α1-blockers, PDE5 inhibitors, and testosterone replacement—alone or in combination—can simultaneously alleviate urinary and sexual symptoms. What is needed now is broader recognition among clinicians, proactive screening, and more robust trials to refine combination therapies.
Addressing LUTSs and ED together transforms management from symptom control to genuine quality-of-life restoration—an outcome every aging man deserves.
FAQ
1. Are lower urinary tract symptoms always linked to erectile dysfunction?
Not always, but the association is strong. Men with moderate to severe LUTSs are up to seven times more likely to have ED compared to men without urinary symptoms.
2. Can PDE5 inhibitors like sildenafil help with urinary symptoms as well as erections?
Yes. PDE5 inhibitors not only improve erections but also reduce LUTS severity, particularly storage symptoms such as urgency and nocturia.
3. Should testosterone therapy be considered in men with LUTSs and ED?
In hypogonadal men, testosterone replacement may improve both urinary and sexual symptoms, especially when combined with PDE5 inhibitors. However, therapy should be personalized and closely monitored.