Introduction
The physiology of penile erection represents one of the most elegant intersections between endocrinology, neurobiology, and vascular medicine. Central to this system are two principal regulators: testosterone, the quintessential androgen, and sildenafil, the archetypal phosphodiesterase type 5 (PDE5) inhibitor. While testosterone is long known as a determinant of male sexual function, and sildenafil is established as a first-line therapy for erectile dysfunction, their interaction in hypogonadal men has remained an area of clinical curiosity.
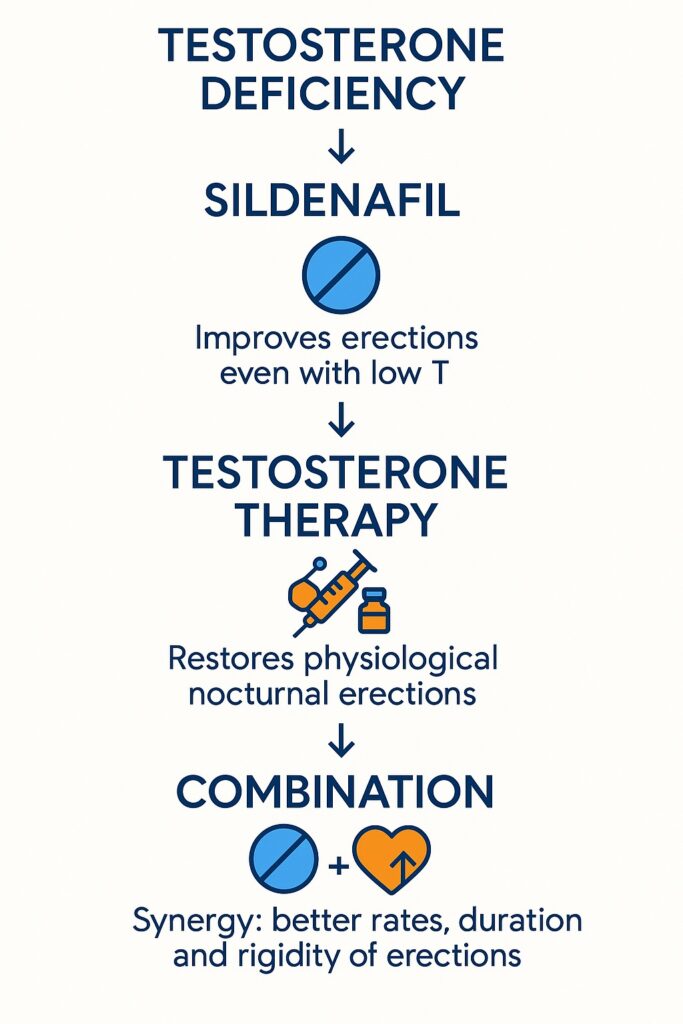
A randomized, placebo-controlled, crossover study has now provided strong evidence that sildenafil significantly improves sleep-related erections even in men with severely reduced testosterone levels. Moreover, when combined with testosterone replacement therapy, sildenafil’s effects are not merely additive but synergistic, producing erectile responses superior to either intervention alone.
This article explores these findings, presenting the pathophysiological background, clinical methodology, outcomes, and broader implications for managing hypogonadal men with erectile dysfunction.
The Androgen Dependence of Male Erections
Androgens, particularly testosterone, exert profound influence on male sexual function. Experimental evidence from animal models demonstrates that castration leads to complete loss of erectile capacity, reversible only with androgen replacement. In men, the relationship is subtler: erections persist at low androgen levels, but their frequency, rigidity, and duration diminish significantly.
Sleep-related erections, also known as nocturnal penile tumescence (NPT), are particularly androgen-dependent. Studies have shown two testosterone thresholds for sexual function: above 350 ng/dL, sleep erections are generally preserved, while psychogenic erections may falter; below 200 ng/dL, both sleep-related and wake erections are consistently impaired. These thresholds provide an invaluable framework for interpreting clinical data in hypogonadal men.
Mechanistically, testosterone supports erectile physiology by maintaining penile smooth muscle integrity, modulating nitric oxide (NO) synthase expression, and regulating PDE5 levels within cavernous tissue. Thus, hypogonadism not only reduces libido but also weakens the very molecular machinery of penile erection.
The Role of the NO–cGMP Pathway
Erectile function relies on relaxation of cavernous smooth muscle, mediated through the nitric oxide (NO)–cGMP pathway. Upon sexual or neural stimulation, NO synthase generates NO, which activates soluble guanylyl cyclase. This increases cGMP, reducing intracellular calcium and producing smooth muscle relaxation with enhanced blood inflow.
PDE5, highly expressed in penile tissue, terminates this signal by hydrolyzing cGMP. Sildenafil selectively inhibits PDE5, prolonging cGMP activity and reinforcing smooth muscle relaxation. It is precisely this mechanism that underpins sildenafil’s efficacy in erectile dysfunction of various origins.
However, in hypogonadal men, the reduced NO availability raises questions: can sildenafil remain effective if the upstream androgen-dependent NO synthesis is impaired? The clinical trial under discussion directly addressed this issue.
Study Design: Hypogonadal and Control Groups
The trial enrolled 24 hypogonadal men (serum testosterone <200 ng/dL) and 24 healthy controls. Hypogonadal participants were tested in two phases:
- Before testosterone replacement therapy (H–T)
- After at least 6 months of testosterone replacement therapy (H+T)
All participants underwent nocturnal penile tumescence and rigidity monitoring (NPTRM) using RigiScan for three consecutive nights. Sildenafil (50 mg) or placebo was administered at bedtime in a randomized crossover design. Parameters assessed included:
- Total number of valid erections
- Total duration of rigidity ≥70%
- Duration of circumference increase ≥30 mm
- Maximum rigidity sustained for ≥3 minutes
- Maximum penile circumference increase
This robust methodology provided a clear window into the androgen–sildenafil interaction, independent of psychological or situational variables that often confound awake erections.
Results: Sildenafil in the Context of Low Testosterone
Before testosterone replacement, hypogonadal men displayed profoundly impaired NPTRM parameters compared to healthy controls. Erections were fewer, shorter, and less rigid.
When sildenafil was administered to these untreated hypogonadal men, all major NPTRM parameters improved significantly compared to placebo. Importantly, their values approached those of healthy controls, demonstrating that sildenafil can indeed recruit the NO–cGMP pathway even when circulating testosterone is severely deficient.
This finding challenges the long-standing assumption that PDE5 inhibitors are ineffective in hypogonadism. Instead, it suggests that although testosterone amplifies NO synthesis, enough basal activity remains for sildenafil to exert therapeutic benefit.
Testosterone Replacement Alone: Restoration of Baseline Function
As expected, testosterone replacement for at least 6 months significantly improved nocturnal erections in hypogonadal men. NPTRM parameters rose to levels indistinguishable from healthy controls on placebo. This confirmed the androgen-dependence of sleep erections and the effectiveness of hormone replacement in restoring physiological erectile function.
Yet, some parameters—such as maximum rigidity—remained modestly improved rather than fully normalized in all subjects, highlighting interindividual variability and the multifactorial nature of erectile function.
Synergy: Testosterone Plus Sildenafil
The most remarkable result of the study was the synergistic effect of combining testosterone with sildenafil. When hypogonadal men on testosterone replacement also received sildenafil at bedtime, NPTRM parameters improved beyond the levels achieved by either therapy alone.
This synergy was particularly striking for the total duration of rigidity and circumference increases, where the combined treatment exceeded the sum of individual effects. Mechanistically, this can be explained as follows:
- Testosterone restores NO synthase activity and enhances PDE5 expression in cavernous tissue.
- Sildenafil inhibits PDE5, capitalizing on the higher enzyme availability induced by testosterone.
- Together, they maximize cGMP levels, producing stronger and longer-lasting erections.
This interaction underscores the principle that effective therapy often requires alignment of both upstream (testosterone-mediated NO production) and downstream (sildenafil-mediated PDE5 inhibition) pathways.
Clinical Implications
The study carries several practical implications for clinical management of hypogonadal men:
- Sildenafil remains effective in hypogonadism: Men with very low testosterone can still benefit from PDE5 inhibitors, challenging the view that androgen replacement is always a prerequisite.
- Testosterone enhances sildenafil efficacy: In men with borderline responses to sildenafil, androgen replacement may convert a partial responder into a full responder.
- Combination therapy is superior: For selected patients, particularly those with confirmed hypogonadism and erectile dysfunction, a combined regimen may represent the most rational therapeutic strategy.
It is equally important to note that not all patients are candidates for testosterone replacement—especially men with prostate cancer or contraindications to androgen therapy. For these individuals, sildenafil alone may still provide meaningful benefit.
Broader Pathophysiological Insights
Beyond clinical practice, this study enriches our understanding of erectile biology:
- Erections are only partially androgen-dependent in humans: Unlike animals, where castration abolishes erections, men retain some erectile capacity even with profoundly reduced testosterone.
- PDE5 expression is androgen-sensitive: Testosterone increases PDE5 levels, paradoxically enhancing the substrate for sildenafil’s inhibitory action.
- Sleep-related erections as a model: Nocturnal erections represent a “pure” form of erectile physiology, largely free from psychological influences, making them an excellent investigative tool.
These insights suggest that erectile function represents a layered physiological process, resilient enough to persist under hormonal deficiency but optimized only when both testosterone and PDE5 pathways are aligned.
Conclusion
The evidence demonstrates that sildenafil improves sleep-related erections in hypogonadal men, even with serum testosterone levels below 200 ng/dL. Testosterone replacement alone restores erectile function to normal, but when combined with sildenafil, the effect is not just additive but synergistic.
For clinicians, this study provides reassurance that PDE5 inhibitors remain viable in hypogonadal states, while also supporting combined therapy for optimal results. For patients, it offers hope that erectile health can be restored even under conditions of hormonal deficiency, provided therapy is individualized and evidence-based.
In broader terms, this work illustrates how pharmacology and endocrinology can reinforce one another, much like the complementary roles of testosterone and sildenafil themselves.
FAQ
1. Can sildenafil work in men with very low testosterone levels?
Yes. This study shows that sildenafil significantly improves sleep-related erections in hypogonadal men, even when testosterone is below 200 ng/dL.
2. Is testosterone replacement always necessary for hypogonadal men with erectile dysfunction?
Not always. Sildenafil alone can improve erections in many hypogonadal men. However, testosterone replacement enhances responsiveness, and combination therapy often yields the best outcomes.
3. Why is the combination of sildenafil and testosterone more effective than either alone?
Because testosterone restores NO production and increases PDE5 expression, while sildenafil inhibits PDE5. The combination maximizes cGMP activity, leading to stronger and longer-lasting erections.