Introduction
Few enzyme families embody both elegance of biochemical design and therapeutic potential as strikingly as cyclic nucleotide phosphodiesterases (PDEs). These enzymes, tasked with the hydrolysis of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), operate as molecular brakes within intracellular signaling cascades. By tightly regulating the spatial and temporal levels of cyclic nucleotides, PDEs orchestrate cellular responses ranging from contraction of smooth muscle to synaptic plasticity in neurons.
Pharmacologists and clinicians have long recognized PDEs as druggable targets. The remarkable success of PDE5 inhibitors in erectile dysfunction and pulmonary arterial hypertension is a case in point: small molecules can reshape intracellular signaling with precision, producing profound physiological outcomes. Yet, PDEs are far more diverse than PDE5 alone. Eleven families and over a hundred isoforms have been identified, each with unique tissue distribution, regulatory mechanisms, and disease associations.
The challenge now lies not in proving PDEs relevant but in harnessing their diversity for therapeutic innovation. This article explores the structure, biology, and pharmacological relevance of PDEs, tracing their role across cardiovascular, pulmonary, neurological, hepatic, oncological, and immunological diseases. It also reflects on the challenges of selectivity, compartmentalization, and safety that define the future of PDE-targeted drug design.
The Architecture of PDE Families
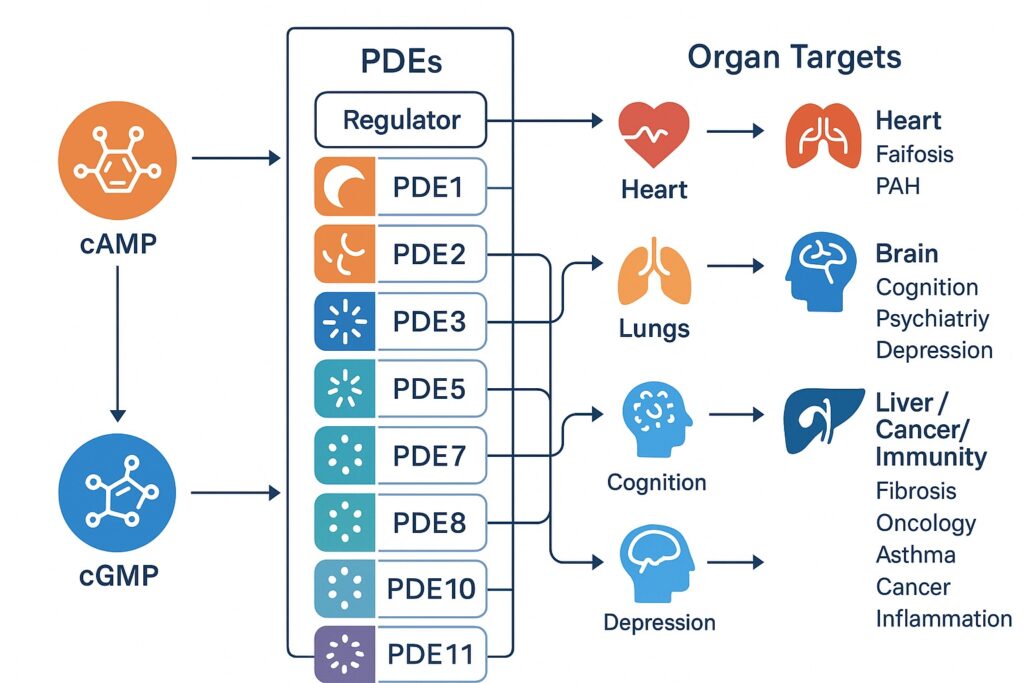
The PDE superfamily is organized into 11 gene families (PDE1–PDE11), producing more than 100 isoforms through alternative splicing. Despite conserved catalytic domains, each family exhibits unique regulatory motifs and substrate preferences.
- PDE1: The only Ca²⁺/calmodulin-dependent family, linking cyclic nucleotide degradation to calcium signaling.
- PDE2: Dual substrate enzymes stimulated allosterically by cGMP, thus integrating cAMP and cGMP pathways.
- PDE3: Notoriously known as “cGMP-inhibited PDEs,” central to cardiac contractility and vascular tone.
- PDE4: A large cAMP-specific family, prominent in inflammatory cells and the central nervous system.
- PDE5: The clinically celebrated cGMP-specific family, underpinning erectile and pulmonary vascular physiology.
- PDE6: Retina-specific enzymes critical for phototransduction.
- PDE7, PDE8, PDE9: More specialized cAMP or cGMP regulators with emerging roles in immunity and cognition.
- PDE10 and PDE11: Dual-substrate enzymes implicated in neuropsychiatric regulation and endocrine signaling.
The interplay of conserved catalytic cores and variable regulatory domains enables PDEs to fine-tune cyclic nucleotide levels in subcellular nanodomains. This compartmentalization is not a minor detail; it explains how cAMP or cGMP can simultaneously mediate opposing processes within the same cell.
PDEs in Cardiovascular Regulation
Cardiac function depends heavily on cAMP and cGMP signaling. Sympathetic stimulation elevates cAMP to increase contractility, while nitric oxide and natriuretic peptides elevate cGMP to promote relaxation. PDEs provide the counterbalance.
PDE3 isoforms are crucial in the heart, hydrolyzing cAMP but inhibited by cGMP. This crosstalk makes PDE3 inhibitors (e.g., milrinone, cilostazol) potent inotrope-vasodilators. Yet, chronic PDE3 inhibition is proarrhythmic, limiting its use to acute heart failure or peripheral vascular disease.
PDE5, although best known in penile smooth muscle, is also expressed in myocardium. PDE5 inhibition has been explored in right heart failure and hypertrophy, though evidence remains mixed. PDE9, another cGMP-selective family, has emerged as a cardiac regulator with potential therapeutic applications.
The lesson from cardiovascular PDE pharmacology is sobering: small molecules can transform physiology but carry risks if chronically disrupting homeostatic balances. Selectivity, dosing, and patient stratification are essential to success.
PDEs in Pulmonary and Vascular Disease
Pulmonary arterial hypertension (PAH) epitomizes the therapeutic relevance of PDE inhibition. Excessive vasoconstriction and vascular remodeling in PAH are countered by elevating cGMP. PDE5 inhibitors—sildenafil, tadalafil, vardenafil—improve pulmonary hemodynamics and exercise capacity, becoming mainstays of PAH therapy.
Beyond PAH, PDE4 inhibitors play an emerging role in airway diseases such as asthma and COPD. By dampening inflammatory cell activation, drugs like roflumilast reduce exacerbations and improve lung function. However, nausea and emesis limit tolerability, underscoring the need for isoform-selective strategies.
PDEs also regulate endothelial barrier integrity and angiogenesis. PDE2 and PDE4 isoforms influence endothelial permeability, while PDE3 and PDE5 modulate vascular tone. As vascular disorders remain leading global killers, fine-tuning cyclic nucleotide pathways could yield new interventions in hypertension, atherosclerosis, and ischemic disease.
PDEs and the Nervous System
Cognition, mood, and behavior are profoundly influenced by cyclic nucleotides. PDEs act as gatekeepers of synaptic signaling, plasticity, and neuronal survival.
PDE4 is heavily expressed in neurons and glia. Preclinical models link PDE4 inhibition to enhanced memory, antidepressant effects, and neuroprotection. Unfortunately, early inhibitors like rolipram caused intolerable nausea. Newer agents, such as allosteric PDE4D modulators, promise improved safety.
PDE10 has attracted attention in psychiatric disorders. Highly expressed in striatal neurons, PDE10 integrates dopaminergic and glutamatergic signaling. Inhibitors were once hailed as potential antipsychotics, though clinical results have been mixed.
PDE9 inhibitors, capable of penetrating the brain, are being investigated for Alzheimer’s disease and age-related cognitive decline. By sustaining cGMP signaling, they may enhance synaptic plasticity and memory consolidation.
Neuropharmacology teaches a cautionary tale: PDE inhibition can boost cognition and mood, but the blood–brain barrier, side effects, and disease complexity demand precision rather than brute-force elevation of cyclic nucleotides.
PDEs in Liver, Cancer, and Inflammation
Beyond heart and brain, PDEs infiltrate nearly every organ system.
In liver fibrosis, PDEs modulate stellate cell activation and extracellular matrix deposition. PDE inhibitors may attenuate fibrogenesis, though clinical translation remains early.
In oncology, PDE5 and PDE10 inhibition has been linked to apoptosis induction and tumor suppression in preclinical models. Intriguingly, repurposing PDE5 inhibitors such as sildenafil for cancer immunotherapy is under exploration, as they may modulate the tumor microenvironment and immune suppression.
In inflammation, PDE4 inhibitors have established proof of concept: by lowering cAMP degradation in leukocytes, they blunt cytokine release and neutrophil activation. This immunomodulatory strategy now extends beyond COPD to dermatology and rheumatology.
Collectively, these observations position PDEs as versatile regulators of pathophysiology, with implications from cirrhosis to oncology.
The Challenge of Selectivity
Designing PDE inhibitors is not simply about finding a molecule that blocks hydrolysis. The true challenge lies in isoform selectivity and compartmentalization.
- Catalytic site conservation: All PDEs share a similar catalytic core, making off-target inhibition likely.
- Allosteric regulation: Families such as PDE2 and PDE5 possess GAF domains that provide unique allosteric sites. Exploiting these could enhance selectivity.
- Nanodomain targeting: PDEs often act within confined subcellular compartments. Disrupting PDE localization, rather than catalytic activity, may offer precision without global side effects.
Innovative strategies now include proteolysis targeting chimeras (PROTACs) to degrade specific PDE proteins, and peptide disruptors to relocate PDEs within nanodomains. Such approaches may overcome the blunt-force limitations of traditional inhibitors.
Future Perspectives
The story of PDEs is far from complete. Several directions define the frontier of research:
- Isoform-specific inhibitors: Moving from family-level to isoform-level precision could reduce side effects and enhance efficacy.
- Hybrid and bifunctional drugs: Combining PDE inhibition with other mechanisms (e.g., anti-inflammatory or vasodilatory) may synergize effects.
- Personalized medicine: Genetic variations in PDE expression or function may guide individualized therapy.
- Repurposing: Well-established PDE inhibitors may find new life in oncology, immunology, and neurology.
The next generation of PDE-targeted drugs will not only rely on chemistry but also on systems biology—mapping signaling networks, subcellular dynamics, and patient-specific molecular landscapes.
Conclusion
Cyclic nucleotide PDEs are more than degradative enzymes; they are molecular architects of signaling precision. Their regulation of cAMP and cGMP cascades underpins critical processes in the heart, lungs, brain, liver, and immune system. Pharmacological inhibition has already revolutionized fields from sexual medicine to respiratory disease, yet much untapped potential remains.
The path forward demands selectivity, creativity, and humility. Selectivity to target the right isoform at the right place. Creativity to exploit allosteric and compartmentalized mechanisms. And humility to accept that cyclic nucleotide biology is complex, demanding nuanced interventions rather than universal fixes.
In this complexity lies opportunity—the chance to transform PDE pharmacology into a cornerstone of 21st-century therapeutics.
FAQ
1. Why are PDEs considered attractive drug targets?
Because they regulate cAMP and cGMP, which control essential processes like vascular tone, cardiac contractility, inflammation, and cognition. Modulating PDEs allows pharmacologists to adjust signaling cascades with precision.
2. Are PDE inhibitors safe for long-term use?
Some, like PDE5 inhibitors, have excellent safety profiles in specific contexts. Others, like PDE3 inhibitors, are limited by proarrhythmic risks. Long-term safety depends on isoform selectivity, tissue distribution, and patient characteristics.
3. What is the future of PDE-targeted therapy?
The future lies in isoform-specific inhibitors, hybrid drugs, and personalized medicine approaches. Novel technologies such as PROTACs and peptide disruptors may enable unprecedented precision in PDE modulation.