Introduction
Sexual health disorders remain among the most underdiagnosed and undertreated areas in modern medicine. In women, particularly, low sexual interest and arousal difficulties are both common and profoundly distressing. The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) consolidated hypoactive sexual desire disorder (HSDD) and female sexual arousal disorder (FSAD) into a single entity: female sexual interest/arousal disorder (FSIAD). This diagnosis acknowledges that desire and arousal are not entirely separable phenomena but closely intertwined. Yet, despite the prevalence and psychosocial burden of FSIAD, pharmacotherapeutic options remain sparse.
One avenue of research has focused on the dual control model of sexual response. This model proposes that sexual function is governed by the dynamic balance between excitation and inhibition systems in the brain. Disruptions in either pathway can lead to clinically significant dysfunction. In women with low sensitivity to sexual stimuli, boosting sexual excitation pathways may help restore normal function. Two agents have been extensively studied in this regard: testosterone and sildenafil. Testosterone enhances central processing of sexual cues, while sildenafil augments genital vasocongestion. The combination aims to address both central and peripheral mechanisms of arousal, thereby offering a synergistic therapeutic approach.
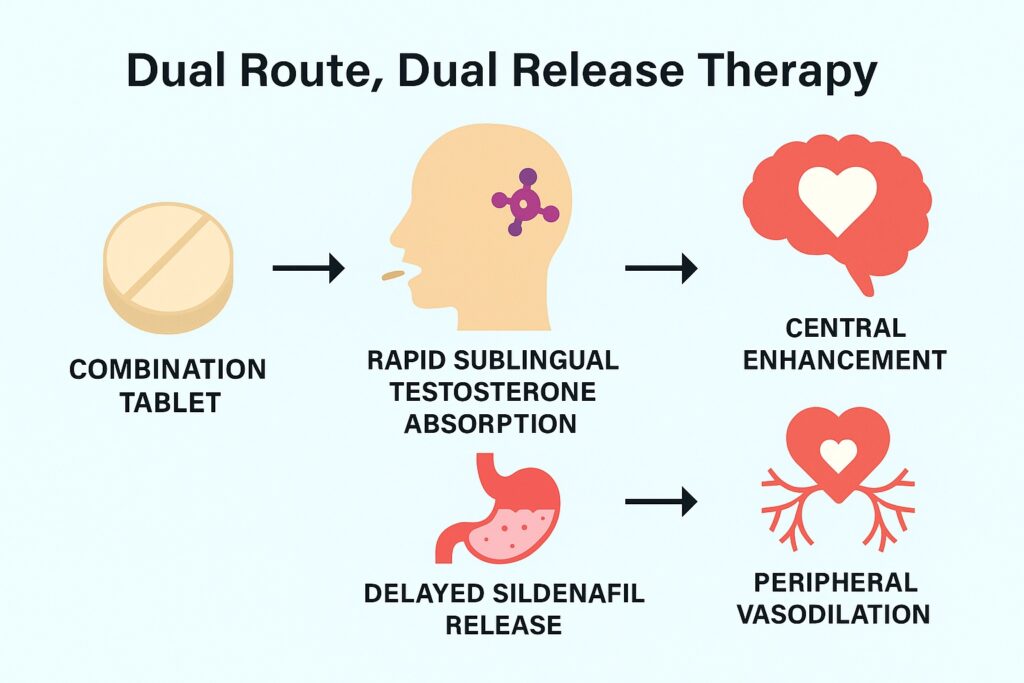
Early proof-of-concept studies administered testosterone sublingually and sildenafil orally, with a 2.5-hour delay between the two. This timing was essential: sublingual testosterone produces rapid pharmacokinetic peaks but delayed behavioral effects, with maximum influence on sexual motivation appearing around 3–6 hours after dosing. Sildenafil, in contrast, exerts its effects rapidly once absorbed. Synchronizing the delayed cognitive effects of testosterone with the genital vascular effects of sildenafil required precise scheduling. The drawback, of course, was practicality—two different dosage forms, separated by hours, invite errors in real-world settings. The solution was a novel pharmaceutical innovation: a dual route/dual release fixed-dose combination tablet.
This article explores the pharmacokinetic comparison between the traditional two-step regimen and this new single-tablet design, unpacking both the scientific rationale and clinical implications.
The Rationale for Combining Testosterone and Sildenafil
To appreciate the innovation, it is important to understand why testosterone and sildenafil make such a compelling pair. Testosterone is not only an androgen regulating libido but also a neuromodulator that heightens brain responsiveness to erotic stimuli. When administered sublingually, plasma levels spike within minutes but return to baseline within two hours. Intriguingly, the behavioral effects persist much longer, reflecting central nervous system modulation rather than sustained systemic exposure. Women receiving sublingual testosterone report heightened sexual interest and motivation several hours after dosing, coinciding with the “window of effect” rather than the pharmacokinetic profile.
Sildenafil, a phosphodiesterase type 5 inhibitor, operates peripherally. By preventing degradation of cyclic GMP, it prolongs nitric oxide–mediated smooth muscle relaxation in genital tissues, leading to increased blood engorgement. In women, this translates into enhanced genital vasocongestion, lubrication, and physical readiness for intercourse. However, sildenafil on its own has shown limited efficacy in treating FSIAD. The issue is not vascular responsiveness per se, but rather the absence of sufficient central arousal to trigger the sexual cascade.
When combined, testosterone primes the brain while sildenafil supports the body. The central enhancement of sexual cues intersects with the peripheral increase in genital responsiveness, yielding a synergistic effect. This scientific marriage, however, required careful timing—hence the traditional two-step regimen with a 2.5-hour delay.
The Problem of Practicality: Why a New Tablet Was Needed
While pharmacologically sound, the original dosing scheme suffered from a glaring limitation: complexity. Patients had to place a sublingual testosterone solution under the tongue, hold it for a minute, swallow, and then—2.5 hours later—remember to take an encapsulated sildenafil tablet. In clinical trials, adherence was monitored closely by research staff, but in daily practice, timing errors and missed doses were inevitable. For a therapy meant to be taken on-demand before anticipated sexual activity, such complexity was impractical and risked undermining effectiveness.
The logical step forward was to design a single tablet that could deliver both agents with the necessary temporal separation. This was no trivial task. The solution was an elegant dual route/dual release system. The outer coating of the tablet dissolves quickly under the tongue, releasing testosterone for rapid sublingual absorption. The inner core, containing sildenafil, is coated with a pH-independent polymer that delays release for approximately 150 minutes, mimicking the traditional 2.5-hour separation. Once the coating ruptures in the gastrointestinal tract, sildenafil is released in a single pulse, not gradually, ensuring a pharmacokinetic profile similar to that of the standalone oral tablet.
Such a formulation preserves the pharmacological synergy while vastly simplifying administration—one tablet, taken once, 3 to 6 hours before anticipated sexual activity. The innovation thus addressed both adherence and practicality, opening the door to broader clinical application.
Study Design: Comparing Prototype vs. Combination Tablet
The pharmacokinetic comparison study was conducted as a randomized, open-label, crossover trial in 12 healthy premenopausal women. Each participant received both formulations on separate occasions:
- Formulation 1 (F1): sublingual testosterone solution (0.5 mg) followed 2.5 hours later by an oral sildenafil tablet (50 mg).
- Formulation 2 (F2): a dual route/dual release fixed-dose tablet containing 0.5 mg sublingual testosterone in the outer coating and 50 mg sildenafil in the delayed-release core.
Blood samples were taken frequently over 26.5 hours to measure plasma concentrations of total testosterone, free testosterone, dihydrotestosterone (DHT), sildenafil, and its primary metabolite N-desmethyl-sildenafil. Pharmacokinetic parameters including maximum concentration (Cmax), time to maximum concentration (Tmax), half-life (t1/2), and area under the curve (AUC) were calculated.
This design allowed for direct within-subject comparison, minimizing inter-individual variability and focusing on differences attributable to formulation.
Key Findings: Testosterone Profiles
Both formulations produced rapid absorption of testosterone, with Tmax around 15 minutes. Half-lives were short (about 37–39 minutes), consistent with previous studies. However, F2 produced significantly higher Cmax and AUC values for total testosterone, free testosterone, and DHT compared with F1.
The reason lies in the delivery system. The solid tablet coating appears to enhance sublingual absorption more efficiently than the liquid solution, likely due to reduced loss from inadvertent swallowing and a stronger concentration gradient in saliva. Importantly, these pharmacokinetic differences do not necessarily translate into exaggerated clinical effects, since behavioral responses to testosterone depend more on central sensitivity windows than on plasma levels. Nonetheless, the higher bioavailability confirms the robustness of the tablet design.
Key Findings: Sildenafil Profiles
For sildenafil, both formulations showed delayed absorption, with Tmax around 3–4 hours, aligning well with the intended design. F2 released sildenafil after about 2.75 hours, closely matching the 2.5-hour delay used in F1.
Cmax and AUC for sildenafil were slightly lower in F2 compared with F1 (about 80% of exposure), likely due to absorption beginning further along the gastrointestinal tract after delayed release. However, these differences were not clinically significant. Sildenafil at 25 mg is effective in men with erectile dysfunction, and the plasma exposure from 50 mg in F2 remained comfortably above therapeutic thresholds. The main metabolite, N-desmethyl-sildenafil, showed comparable kinetics between the two formulations.
Overall, F2 successfully replicated the pharmacokinetic timing of F1 while simplifying administration into a single dosage form.
Clinical Implications: Toward Practical Sexual Medicine
The results validate the dual route/dual release tablet as a clinically feasible alternative to the complex two-step regimen. By combining both agents into one formulation, it minimizes user error, increases adherence, and simplifies instructions for patients. For a condition as sensitive as FSIAD, where stigma and reluctance to seek help already limit treatment uptake, a practical and discreet option could make a meaningful difference.
Moreover, the combination reflects a broader trend toward personalized sexual medicine. Instead of “one drug fits all,” therapy is increasingly tailored to the underlying mechanism of dysfunction. For women with low sensitivity to sexual stimuli, the testosterone–sildenafil combination addresses both central and peripheral elements of arousal. For those with heightened sexual inhibition, alternative combinations (e.g., testosterone with buspirone) may be more appropriate. Such stratification mirrors personalized approaches already common in oncology and psychiatry.
Another advantage of the single-tablet design is scalability. Large-scale clinical trials, required for regulatory approval, become more practical when dosing complexity is minimized. In daily practice, a one-tablet solution enhances real-world effectiveness, which depends not only on pharmacology but also on adherence and usability.
Limitations and Considerations
While promising, the study had limitations. The sample size was small (n=12), limiting generalizability. Participants were healthy premenopausal women, most on hormonal contraception; results may differ in postmenopausal women or those with comorbidities. Testosterone exhibits natural diurnal and cyclical variations not accounted for here. Furthermore, only pharmacokinetic parameters were studied; clinical outcomes such as sexual satisfaction, frequency of intercourse, and psychological well-being were not directly measured.
Despite these caveats, the findings provide strong proof of principle. The dual release tablet delivers testosterone and sildenafil in a manner closely aligned with the original regimen, supporting further clinical testing in larger and more diverse populations.
Conclusion
The development of a dual route/dual release testosterone–sildenafil tablet represents an important step in sexual medicine. By combining rapid sublingual testosterone absorption with delayed sildenafil release, it achieves the necessary pharmacological synchrony while simplifying administration. The formulation demonstrated higher testosterone bioavailability and comparable sildenafil kinetics to the prototype regimen, fulfilling its design goals.
This innovation exemplifies the evolving philosophy of treating sexual dysfunction—not merely addressing symptoms, but tailoring therapy to the neuroendocrine and vascular underpinnings of the disorder. If validated in larger trials, the combination tablet may offer women with FSIAD a practical, on-demand, and effective treatment option, advancing both science and patient care.
FAQ
1. Why combine testosterone with sildenafil for women?
Testosterone enhances brain sensitivity to sexual cues, while sildenafil improves genital blood flow. Together, they target both central and peripheral mechanisms of arousal, offering synergistic benefits for women with FSIAD.
2. Is the new dual release tablet more effective than taking the drugs separately?
Pharmacokinetically, the single tablet mimics the separate two-step regimen while increasing testosterone absorption and maintaining sildenafil exposure. The main advantage is practicality—one tablet instead of two doses separated by hours.
3. Does higher testosterone absorption mean greater side effects?
Not necessarily. Behavioral effects of testosterone depend more on central sensitivity than on plasma concentration. In this study, testosterone exposure was higher with the tablet, but still within safe ranges. Further clinical trials will clarify safety and tolerability.